Aluminum in Vaccines: What You Need To Know
 1. WHAT IS ALUMINUM?
1. WHAT IS ALUMINUM?
Aluminum is a silvery-white, moldable, and durable light metal. These qualities make it useful in numerous industries and products, including machinery, construction, storage, cookware, eating utensils, textiles, dyes, and cosmetics. Aluminum is also the most abundant metal in the Earth’s crust, and virtually all aluminum in the environment is in soil. However, aluminum is not naturally found in significant amounts in living organisms (such as plants and animals), and aluminum has no known biological function. During the past century, aluminum usage in certain products has led to higher human exposure. The greatest sources of such exposure are aluminum-containing foods (e.g., baking powder, processed foods, infant formulas, etc.), medical products (e.g., antiperspirants, antacids, etc.), allergy shots, and vaccines.1-3
 2. WHY IS ALUMINUM IN VACCINES?
2. WHY IS ALUMINUM IN VACCINES?
Certain vaccines use aluminum compounds (i.e., aluminum hydroxide and aluminum phosphate) as adjuvants, ingredients that enhance the immune response to an antigen (foreign substance).4,5 The U.S. Food and Drug Administration (FDA) states that if some vaccines did not include aluminum, the immune response they trigger may be diminished.6
 3. WHICH VACCINES CONTAIN ALUMINUM?
3. WHICH VACCINES CONTAIN ALUMINUM?
The following vaccines contain aluminum and are administered to infants, children and adolescents (Fig. 1):
- Hepatitis B (HepB)
- Diphtheria, tetanus, and pertussis (whooping cough) (DTaP and Tdap)
- Haemophilus influenzae type b (PedvaxHIB)
- Pneumococcal (PCV)
- Hepatitis A (HepA)
- Human papillomavirus (HPV)
- Meningococcal B (MenB)
 4. IS EXPOSURE TO ALUMINUM SAFE?
4. IS EXPOSURE TO ALUMINUM SAFE?
The FDA has considered aluminum to be generally recognized as safe (GRAS) since 1975.9 However, before 1990, the technology did not exist to accurately detect small quantities of aluminum administered to subjects in scientific studies.10 Consequently, the amount of aluminum that could be absorbed before the onset of negative effects was not known.
Since 1990, due to advancements in technology, small amounts of aluminum that remain in the human body have been observed to interfere with a variety of cellular and metabolic processes in the nervous system and in tissues of other parts of the body.1,10,11 The greatest negative effects of aluminum have been observed in the nervous system and range from motor skill impairment to encephalopathy (altered mental state, personality changes, difficulty thinking, loss of memory, seizures, coma, and more).2,12
The U.S. Department of Health and Human Services (HHS) recognizes aluminum as a known neurotoxin.2 In addition, the FDA has warned about the risks of aluminum toxicity in infants and children.13
 5. HOW MUCH ORAL ALUMINUM IS UNSAFE?
5. HOW MUCH ORAL ALUMINUM IS UNSAFE?
In 2008, the Agency for Toxic Substances and Disease Registry (ATSDR), a division of HHS, used studies of the neurotoxic effects of aluminum to determine that no more than 1 milligram (mg) (1,000 micrograms [mcg]) of aluminum per kilogram (kg) of body weight should be taken orally per day to avoid aluminum’s negative effects.2
 6. HOW MUCH INJECTED ALUMINUM IS UNSAFE?
6. HOW MUCH INJECTED ALUMINUM IS UNSAFE?
To determine the amount of aluminum that can be safely injected requires a conversion of the ATSDR oral aluminum limit. The ATSDR oral aluminum limit is based on 0.1% of oral aluminum being absorbed into the bloodstream, as the digestive tract blocks nearly all oral aluminum (Fig. 2a).2 In contrast, aluminum injected intramuscularly bypasses the digestive tract, and 100% of aluminum may be absorbed into the bloodstream over time (i.e., the proportion of absorbed aluminum is 1,000 times greater). To account for these different absorption amounts, the ATSDR oral aluminum limit must be divided by 1,000. This conversion results in an ATSDR-derived bloodstream aluminum limit of 1 mcg of aluminum (0.1% of 1,000 mcg) per kg of body weight per day (Fig. 2b). Consequently, to avoid the neurotoxic effects of aluminum, no more than 1 mcg of aluminum per kg of body weight should enter the bloodstream on a daily basis. Figure 3 shows the ATSDR-derived bloodstream aluminum limit for infants of various ages based on their weight.
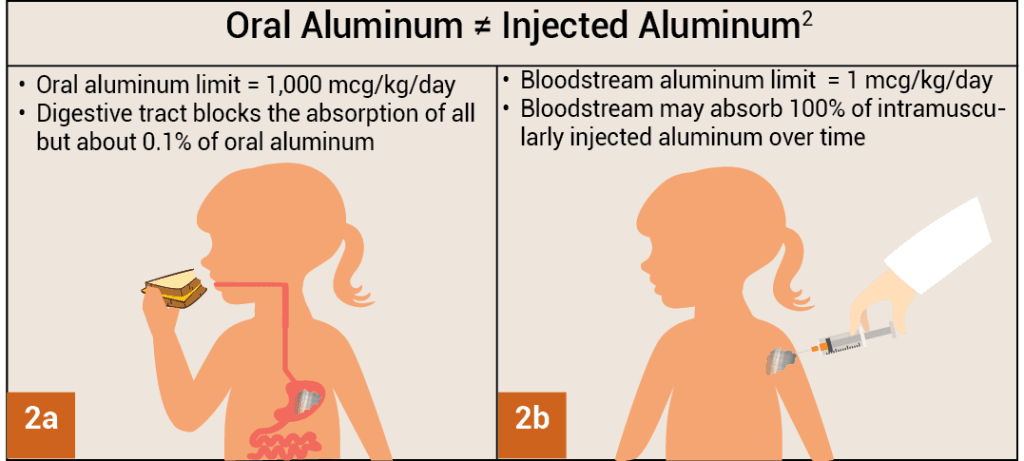
Figures 2a, 2b: When taken orally, only about 0.1% of aluminum is able to enter the bloodstream through the digestive tract (2a). In contrast, when intramuscularly injected, the proportion of aluminum that enters the bloodstream over time is 1,000 times greater (100%) because the digestive tract is bypassed (2b).
 7. HOW MUCH ALUMINUM IS IN VACCINES?
7. HOW MUCH ALUMINUM IS IN VACCINES?
The amount of aluminum in vaccines varies.16 In 1968, the federal government set the limit for the amount of aluminum in vaccines to 850 mcg per dose based on the amount of aluminum needed to make certain vaccines effective.6,17 Consequently, the amount of aluminum in aluminum-containing childhood vaccines ranges from 125 to 850 mcg per dose. Figure 4 shows the aluminum content of one dose of various vaccines administered to children.7,18,19
 8. HAVE ANY STUDIES COMPARED THE AMOUNT OF ALUMINUM IN VACCINES TO THE ATSDR-DERIVED LIMIT?
8. HAVE ANY STUDIES COMPARED THE AMOUNT OF ALUMINUM IN VACCINES TO THE ATSDR-DERIVED LIMIT?
A recent study that intended to compare the amount of aluminum in vaccines to the ATSDR-derived bloodstream limit was published in 2011.20 However, this study incorrectly based its calculations on 0.78% of oral aluminum being absorbed into the bloodstream rather than the value of 0.1% used by the ATSDR in its computations.21,22 As a result, the 2011 study assumed that nearly 8 (0.78%/0.1%) times more aluminum can safely enter the bloodstream, and this led to an incorrect conclusion.
 9. IS EXPOSURE TO ALUMINUM FROM VACCINES SAFE?
9. IS EXPOSURE TO ALUMINUM FROM VACCINES SAFE?
Vaccines are injected intramuscularly, and the rate at which aluminum from vaccines migrates from human muscle to the bloodstream is not known. Studies in animals suggest that it can take from a couple of months to more than a year for aluminum from vaccines to enter into the bloodstream, due to multiple variables.23-25 Because the cumulative aluminum exposure from vaccines in children less than 1 year old exceeds the ATSDR-derived daily limit by several hundreds (Figs. 3 and 4), the limit would still be exceeded if aluminum from vaccines entered the bloodstream over the course of about a year. Moreover, studies have shown that aluminum from vaccines is absorbed by immune cells that travel to distant parts of the body, including the brain.26
Studies have also shown that adverse effects of aluminum in vaccines may not be restricted to neurological conditions. A study published in Academic Pediatrics found that asthma occurred in 1 in 183 vaccinated children for every 1 mg (1,000 mcg) increase in aluminum exposure.27
Clinical trials including subjects of age 9 or older, where 13,023 subjects were injected with an aluminum compound and 594 were injected with a saline placebo, observed 18 serious systemic adverse reactions (1 in 724) in the aluminum group and none in the saline group.28 Safety studies comparing children younger than age 9 vaccinated with aluminum-containing vaccines to young children not vaccinated with such vaccines have not been conducted.

Figure 3: This chart shows the aluminum limit for infants of various ages, as derived from the Agency for Toxic Substances and Disease Registry, a division of the U.S. Department of Health and Human Services. The limit indicates that no more than 1 mcg of aluminum per kg of body weight should enter the bloodstream on a daily basis to avoid the neurotoxic effects of aluminum.
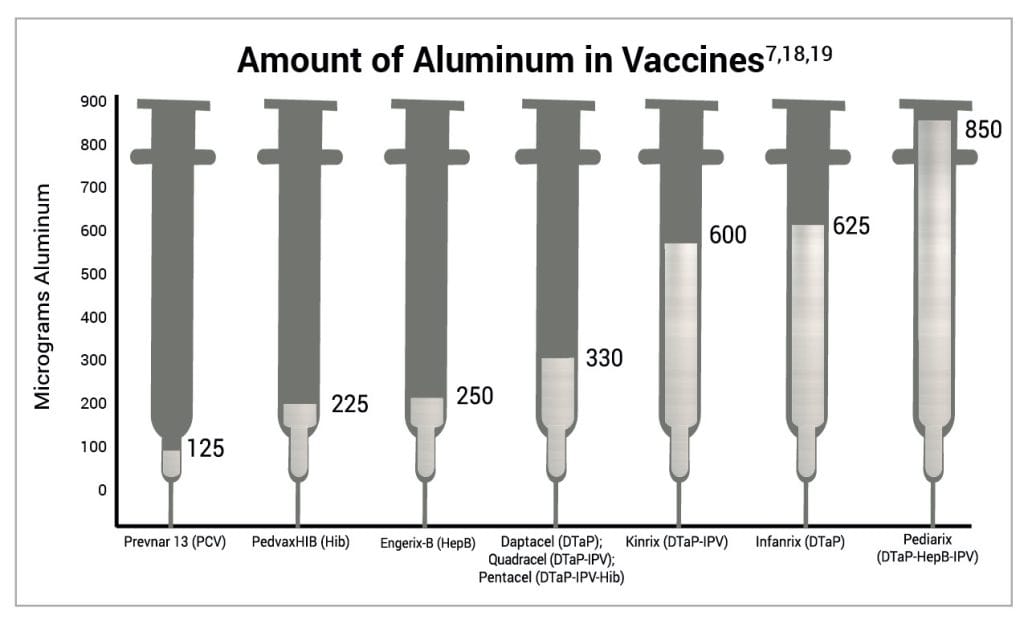
Figure 4: This graph shows the aluminum content of one dose of various vaccines administered to children. The administration of one dose each of Prevnar 13, PedvaxHIB, Engerix-B, and Infanrix at one visit delivers 1,225 mcg of aluminum. PCV, Hib, HepB, and DTaP vaccines are administered multiple times by 6 months of age. The rate at which aluminum from vaccines migrates from human muscle to the bloodstream is not known.
REFERENCES
- American Academy of Pediatrics, Committee on Nutrition. Aluminum toxicity in infants and children. Pediatrics. 1996 Mar;97(3):413. https://publications.aap.org/pediatrics/article-abstract/97/3/413/60076/Aluminum-Toxicity-in-Infants-and-Children.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for aluminum. Washington, D.C.: U.S. Department of Health and Human Services; 2008.3, 13-24, 145, 171-7, 208. https://www.atsdr.cdc.gov/toxprofiles/tp22.pdf.
- Yokel RA. Aluminum in food—the nature and contribution of food additives. In: El-Samragy Y, editor. Food additive. Rijeka (Croatia): InTech; 2012. 203-28. https://www.intechopen.com/chapters/28917.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009 Apr;9(4):287. https://pubmed.ncbi.nlm.nih.gov/19247370/.
- Volk VK, Bunney WE. Diphtheria immunization with fluid toxoid and alum-precipitated toxoid. Am J Public Health Nations Health. 1942 Jul;32(7):690-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1527016/.
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines—U.S. perspective. Vaccine. 2002 May 31;20 Suppl 3:S18-22. https://pubmed.ncbi.nlm.nih.gov/12184360/.
- U.S. Food and Drug Administration. Silver Spring (MD): U.S. Food and Drug Administration. Vaccines licensed for use in the United States; [updated 2018 Feb 14; cited 2018 Feb 27]. https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/Ucm093833.htm.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2022; 2022 Feb 17 [cited 2022 Dec 4]. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
- U.S. Food and Drug Administration. Silver Spring (MD): U.S. Food and Drug Administration. SCOGS (Select Committee on GRAS Substances); [cited 2024 Jul 15]. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS.
- Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit. 2004;6:376,392. https://www.ncbi.nlm.nih.gov/pubmed/15152306.
- Poole RL, Pieroni KP, Gaskari S, Dixon TK, Park KT, Kerner JA. Aluminum in pediatric parenteral nutrition products: measured versus labeled content. J Pediatr Pharmacol Ther. 2011;16(2):92-7. https://www.ncbi.nlm.nih.gov/pubmed/22477831.
- Sedman A. Aluminum toxicity in childhood. Pediatr Nephrol. 1992 Jul;6(4):383-93. https://www.ncbi.nlm.nih.gov/pubmed/1498007.
- U.S. Food and Drug Administration, Department of Health and Human Services. Rules and regulations. Fed Regist. 2003 Jun 9;68(110):34286. https://www.govinfo.gov/content/pkg/FR-2003-06-09/pdf/03-14140.pdf.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. National Center for Health Statistics: Data table for boys length-for-age and weight-for-age charts; [cited 2019 April 2]. https://physiciansforinformedconsent.org/cdc-growth-chart-boys-length-weight.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. National Center for Health Statistics: Data table for girls length-for-age and weight-for-age charts; [cited 2019 April 2]. https://physiciansforinformedconsent.org/cdc-growth-chart-girls-length-weight.
- U.S. Food and Drug Administration, Department of Health and Human Services. Revision of the requirements for constituent materials. Final rule. Fed Regist. 2011 Apr 13;76(71):20513-8. https://www.federalregister.gov/documents/2011/04/13/2011-8885/revision-of-the-requirements-for-constituent-materials.
- Office of the Federal Register, National Archives and Records Service, General Services Administration. Rules and regulations. Fed Regist. 1968 Jan; 33(6):369. https://tile.loc.gov/storage-services/service/ll/fedreg/fr033/fr033006/fr033006.pdf.
-
GlaxoSmithKline Biologics. Rixensart, Belgium: GlaxoSmithKline. Kinrix (diphtheria and tetanus toxoids and acellular pertussis adsorbed and inactivated poliovirus vaccine); [cited 2023 Apr 9]. http://wayback.archive-it.org/7993/20170723032435/https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM241453.pdf; as of November 2022, the FDA posted an updated package insert, with decreased aluminum content, to its Kinrix product information webpage. No documentation is available explaining the change in the aluminum content. PIC has filed a FOIA request to examine the discrepancy.
-
GlaxoSmithKline Biologics. Rixensart, Belgium: GlaxoSmithKline. Infanrix (diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed); [cited 2023 Feb 23]. http://wayback.archive-it.org/7993/20170723024611/https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM124514.pdf; as of November 2022, the FDA posted an updated package insert, with decreased aluminum content, to its Infanrix product information webpage. No documentation is available explaining the change in the aluminum content. PIC has filed a FOIA request to examine the discrepancy.
- Mitkus RJ, King DB, Hess MA, Forshee RA, Walderhaug MO. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. 2011 Nov 28;29(51):9538-43. https://pubmed.ncbi.nlm.nih.gov/22001122/.
- Miller S, Physicians for Informed Consent. Erratum in ‘Updated aluminum pharmacokinetics following infant exposures through diet and vaccination.’ In: ResearchGate. Berlin (Germany): ResearchGate GmbH; 2020 Mar 6 [cited 2020 Mar 6]. https://www.researchgate.net/publication/51718934_Updated_Aluminum_pharmacokinetics_following_infant_exposures_through_diet_and_vaccines/comments.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Erratum in ‘Updated aluminum pharmacokinetics following infant exposures through diet and vaccination’; [cited 2020 Mar 6]. https://physiciansforinformedconsent.org/mitkus-2011-erratum/.
- Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC, Dandashli EA. In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine. 1997 Aug-Sept;15(12-13):1314-8. https://pubmed.ncbi.nlm.nih.gov/9302736/.
- Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine. 2005 Feb 3;23(11):1359-67. https://pubmed.ncbi.nlm.nih.gov/15661384/.
- Weisser K, Göen T, Oduro JD, Wangorsch G, Hanschmann KO, Keller-Stanislawski B. Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Arch Toxicol. 2019 Oct;93(10):2787-96. https://pubmed.ncbi.nlm.nih.gov/31522239/.
- Masson JD, Crépeaux G, Authier FJ, Exley C, Gherardi RK. Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants. J Inorg Biochem. 2018 Apr;181:87-95. https://pubmed.ncbi.nlm.nih.gov/29307441/.
- Daley MF, Reifler LM, Glanz JM, Hambidge SJ, Getahun D, Irving SA, Nordin JD, McClure DL, Klein NP, Jackson ML, Kamidani S, Duffy J, DeStefano F. Association between aluminum exposure from vaccines before age 24 months and persistent asthma at age 24 to 59 months. Acad Pediatr. 2022 Sep 27:S1876-2859(22)00417-X. Epub ahead of print. https://pubmed.ncbi.nlm.nih.gov/36180331/; the study found a 26% increased risk of asthma for every 1 mg increase in aluminum exposure. Because the overall risk of asthma in children without eczema was 2.1%, an increased risk of 26% results in an absolute risk of 0.546% (2.1% times 26%, or 1 in 183).
- Merck. Whitehouse Station (NJ): Merck and Co., Inc. Gardasil; revised 2015 Apr [cited 2024 Jul 10]. 7. https://www.fda.gov/files/vaccines, blood & biologics/published/Package-Insert—Gardasil.pdf.
Published 2020 Aug; updated 2024 Dec

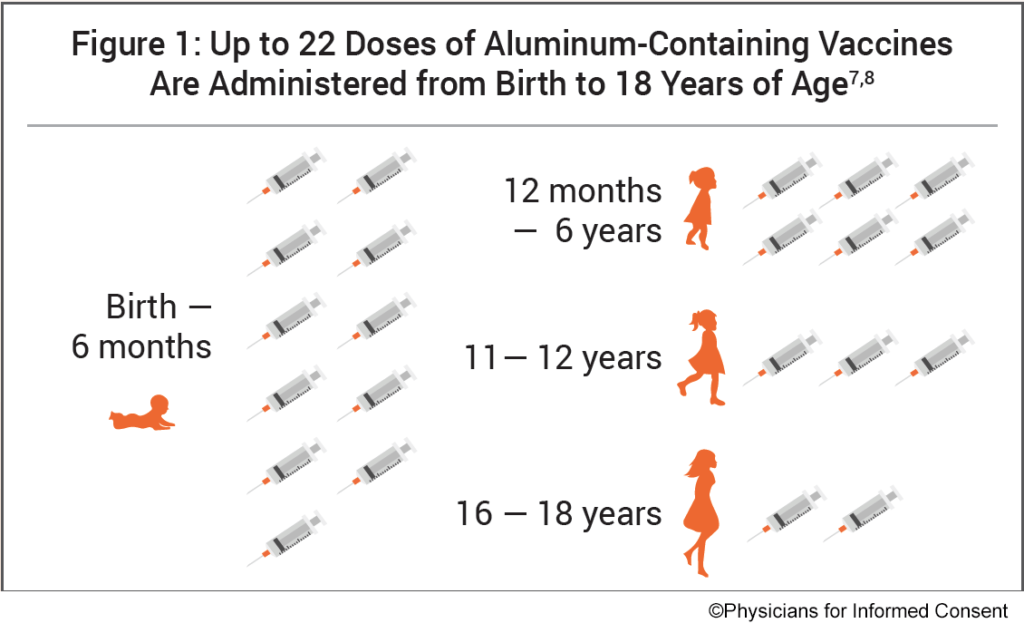


[…] i medici per il consenso informato, vengono somministrate fino a 22 dosi di vaccini contenenti alluminio dalla nascita fino ai 18 anni […]
[…] to Physicians for Informed Consent, up to 22 doses of aluminum-containing vaccines are administered from birth to 18 years of […]
[…] acordo com os Médicos pelo Consentimento Informado, até 22 doses de vacinas contendo alumínio são administradas desde o nascimento até os 18 anos […]
[…] to Physicians for Informed Consent, up to 22 doses of aluminum-containing vaccines are administered from birth to 18 years […]
[…] Physicians for Informed Consent, dalla nascita fino ai 18 anni di età vengono somministrate fino a 22 dosi di vaccini contenenti […]
[…] Physicians for Informed Consent, până la 22 de doze de vaccinuri care conțin aluminiu sunt administrate de la naștere până la […]