Haemophilus Influenzae Type B (Hib) – Vaccine Risk Statement (VRS)
Hib Vaccine (Haemophilus Influenzae Type B)
Is It Safer Than Hib?
What Is the Hib Vaccine?
The first Haemophilus influenzae type b (Hib) vaccine was a pure polysaccharide vaccine that was introduced in the U.S. in 1985, but it had low efficacy and was replaced with the Hib conjugate vaccine in 1987.1 The Hib vaccine has significantly reduced the incidence of reported (i.e., noticeable) cases of Hib infections; however, studies have observed that mass vaccination may lead to an increase in the prevalence of non-type b Haemophilus influenzae strains.2,3
What Are Side Effects of the Hib Vaccine?
Common side effects of the Hib vaccine include fever, diarrhea, vomiting, loss of appetite, ear infection, rash, drowsiness, and upper respiratory infection.4,5 A more serious potential side effect is seizure, which may occur in about 1 in 1,098 children vaccinated with Hib vaccine.6-9 Also, a study published in Autoimmunity in 2002 observed an increased risk of type 1 diabetes of 1 in 1,852 among children who received 4 doses of the Hib vaccine.10
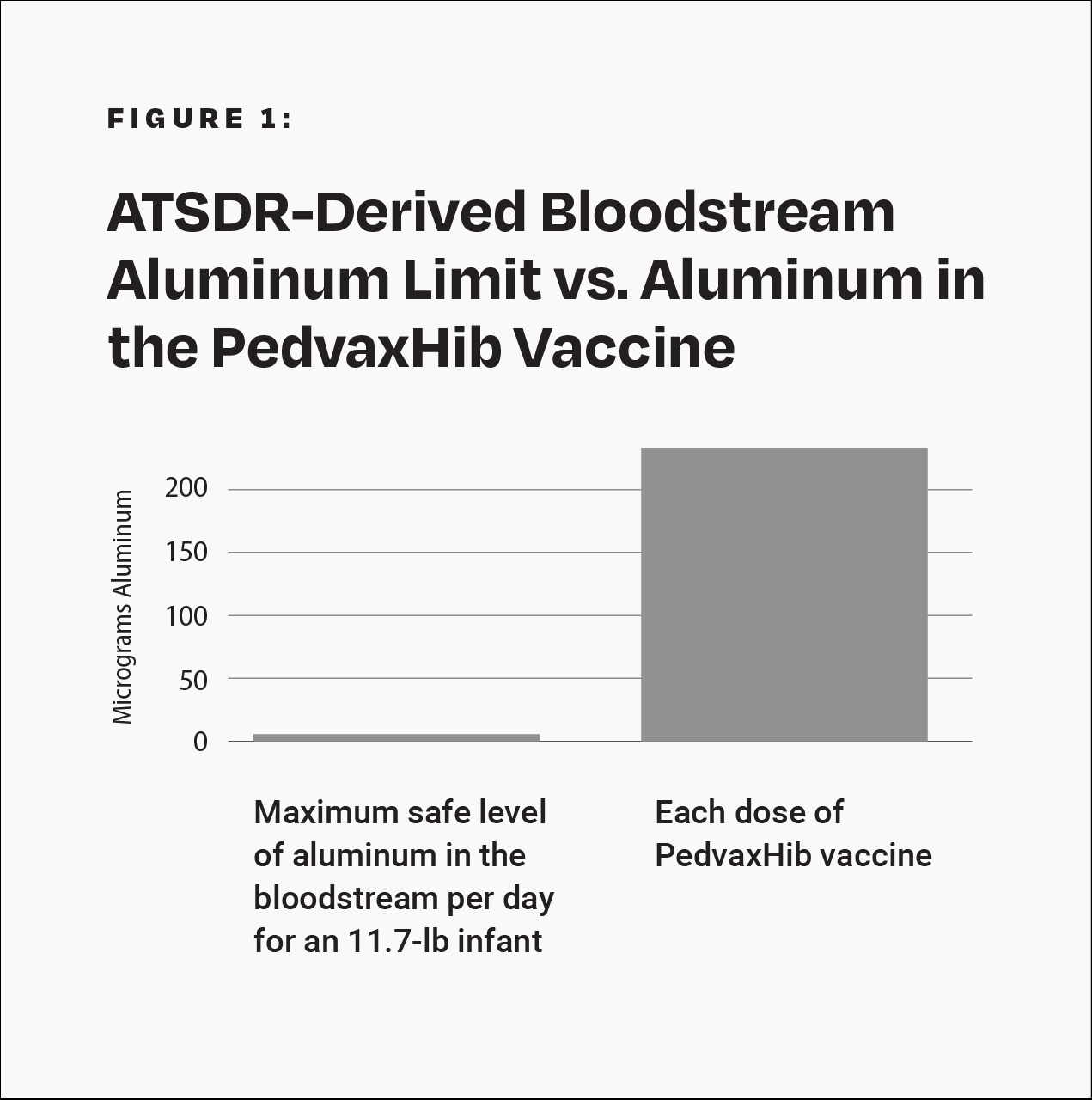
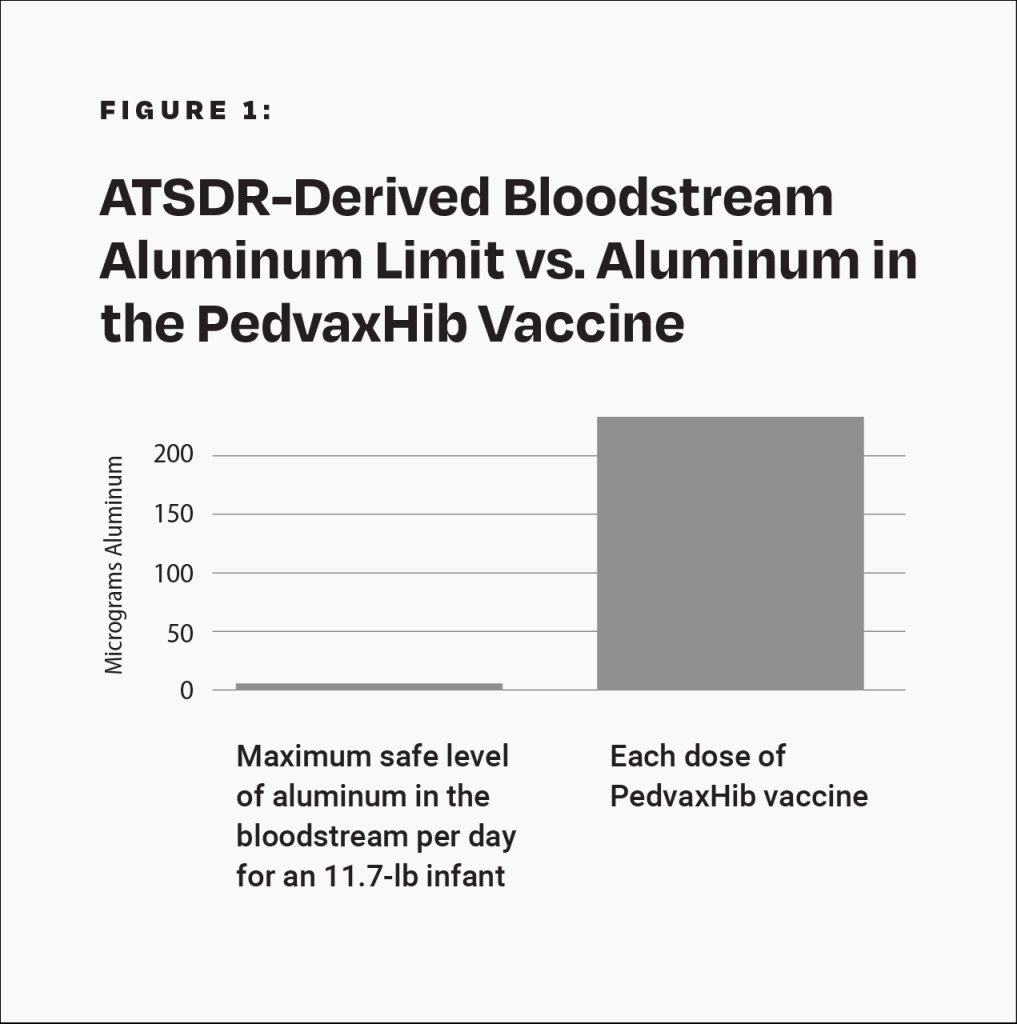
Although severe adverse events, such as transverse myelitis, Guillain-Barré syndrome, thrombocytopenia, and sudden infant death syndrome, have been observed following Hib vaccination, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relation between Hib vaccines” and those conditions.11 The PedvaxHib vaccine contains 225 mcg of aluminum.4 This amount is more than 40 times greater than the maximum safe level of aluminum in the bloodstream per day for an 11.7-pound (5.3-kilogram) infant, derived from the Agency for Toxic Substances and Disease Registry (ATSDR), a division of the U.S. Department of Health and Human Services (HHS) (Fig. 1).12 Additionally, the manufacturer’s package insert states that the Hib vaccine “has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility.”4
How Are Risks of Vaccine Side Effects Measured?
Methods to measure vaccine risks include surveillance systems, clinical trials, and epidemiological studies.
How Accurate Is Surveillance of Adverse Events from the Hib Vaccine?
The government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 101 cases of permanent disability or death from the Hib vaccine are reported to VAERS annually.13 However, VAERS is a passive reporting system — authorities do not actively search for cases and do not actively remind doctors and the public to report cases. These limitations can lead to significant underreporting.14 The Centers for Disease Control and Prevention (CDC) states, “VAERS receives reports for only a small fraction of actual adverse events.”15 Indeed, as few as 1% of serious side effects from medical products are reported to passive surveillance systems.16 In addition, VAERS reports are not proof that a side effect occurred, as the system is not designed to thoroughly investigate all cases.17 As a result, VAERS does not provide an accurate count of Hib vaccine side effects.
How Accurate Are Clinical Trials of the Hib Vaccine?
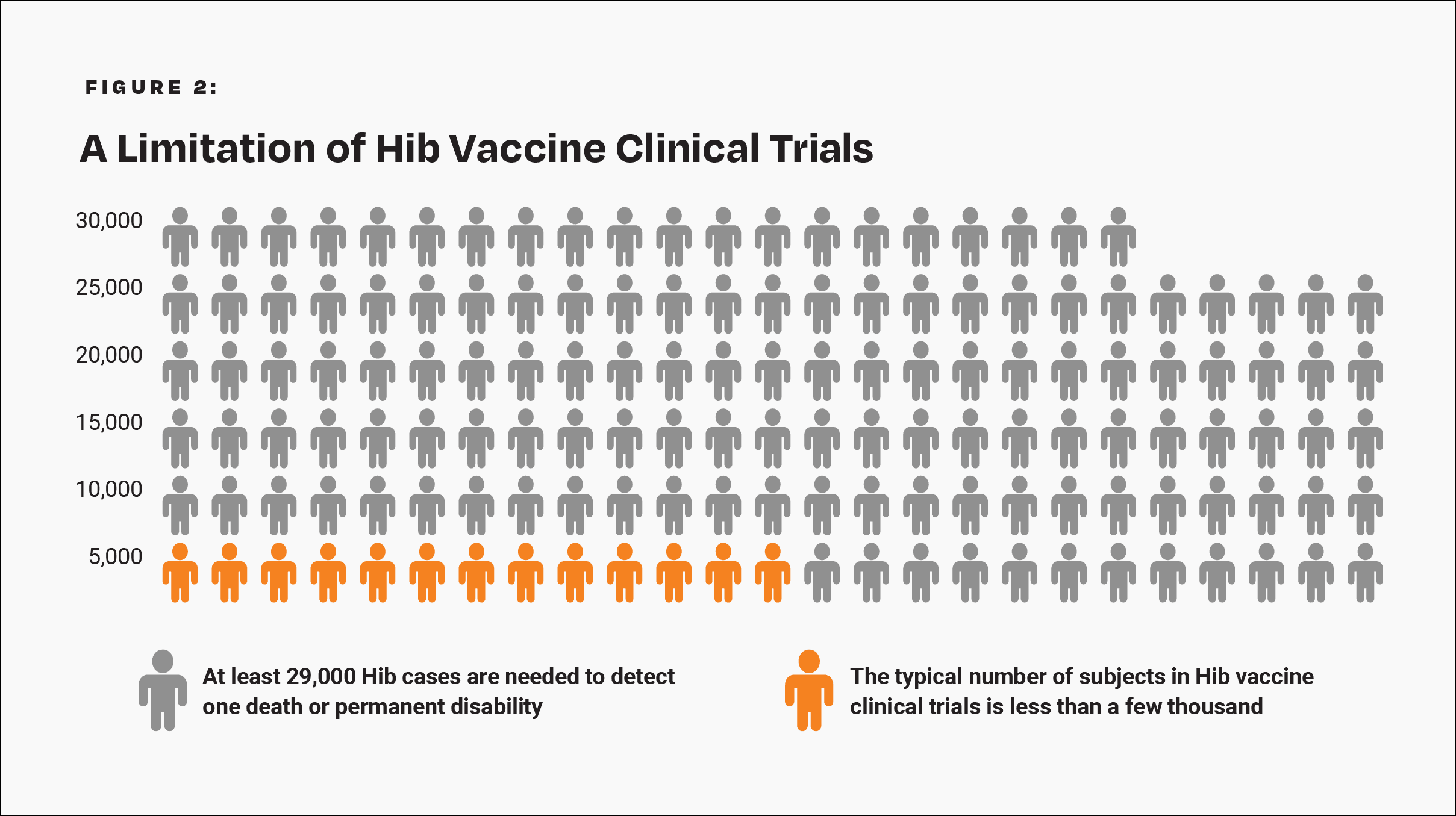
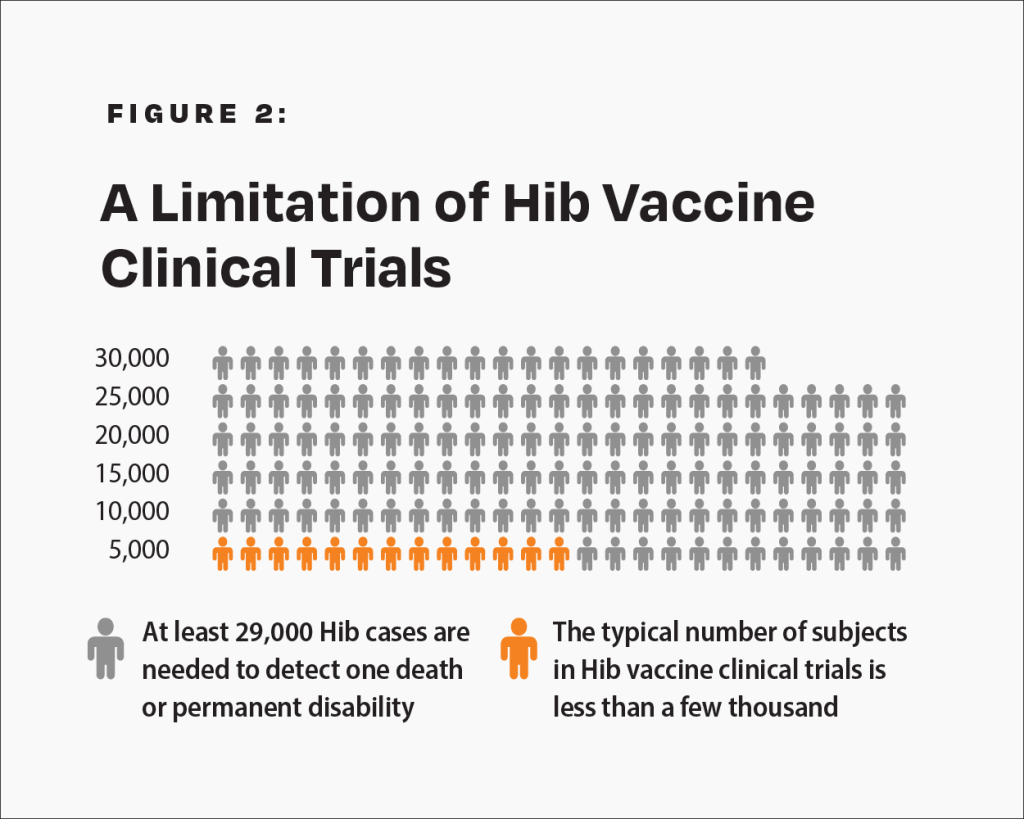
The CDC states, “Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.”14 In the pre-vaccine era, annually, permanent disability or death from Hib occurred in about 1 in 143,000 children under age 5 who were breastfed exclusively (i.e., breastfed alone without formula) for 13 weeks or more.18 Therefore, about 1 in 29,000 such children suffered permanent disability or death from Hib in a 5-year span. A few thousand subjects in clinical trials are not enough to prove that the Hib vaccine causes less permanent disability or death than Hib in children who are breastfed exclusively for 13 weeks or more (Fig. 2).
What Is the Hib Vaccine?
The first Haemophilus influenzae type B (Hib) vaccine was a pure polysaccharide vaccine that was introduced in the U.S. in 1985, but it had low efficacy and was replaced with the Hib conjugate vaccine in 1987.1 The Hib vaccine has significantly reduced the incidence of reported (i.e., noticeable) cases of Hib infections; however, studies have observed that mass vaccination may lead to an increase in the prevalence of non-type B Haemophilus influenzae strains.2,3
What Are Side Effects of the Hib Vaccine?
Common side effects of the Hib vaccine include fever, diarrhea, vomiting, loss of appetite, ear infection, rash, drowsiness, and upper respiratory infection.4,5 A more serious potential side effect is seizure, which may occur in about 1 in 1,098 children vaccinated with Hib vaccine.6-9 Also, a study published in Autoimmunity in 2002 observed an increased risk of type 1 diabetes of 1 in 1,852 among children who received 4 doses of the Hib vaccine.10
Although severe adverse events, such as transverse myelitis, Guillain-Barré syndrome, thrombocytopenia, and sudden infant death syndrome, have been observed following Hib vaccination, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relation between Hib vaccines” and those conditions.11 The PedvaxHib vaccine contains 225 mcg of aluminum.4 This amount is more than 40 times greater than the maximum safe level of aluminum in the bloodstream per day for an 11.7-pound (5.3-kilogram) infant, derived from the Agency for Toxic Substances and Disease Registry (ATSDR), a division of the U.S. Department of Health and Human Services (HHS) (Fig. 1).12 Additionally, the manufacturer’s package insert states that the Hib vaccine “has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility.”4
How Are Risks of Vaccine Side Effects Measured?
Methods to measure vaccine risks include surveillance systems, clinical trials, and epidemiological studies.
How Accurate Is Surveillance of Adverse Events from the Hib Vaccine?
The government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 101 cases of permanent disability or death from the Hib vaccine are reported to VAERS annually.13 However, VAERS is a passive reporting system — authorities do not actively search for cases and do not actively remind doctors and the public to report cases. These limitations can lead to significant underreporting.14 The Centers for Disease Control and Prevention (CDC) states, “VAERS receives reports for only a small fraction of actual adverse events.”15 Indeed, as few as 1% of serious side effects from medical products are reported to passive surveillance systems.16 In addition, VAERS reports are not proof that a side effect occurred, as the system is not designed to thoroughly investigate all cases.17 As a result, VAERS does not provide an accurate count of Hib vaccine side effects.
How Accurate Are Clinical Trials of the Hib Vaccine?
The CDC states, “Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.”14 In the pre-vaccine era, annually, permanent disability or death from Hib occurred in about 1 in 143,000 children under age 5 who were breastfed exclusively (i.e., breastfed alone without formula) for 13 weeks or more.18 Therefore, about 1 in 29,000 such children suffered permanent disability or death from Hib in a 5-year span. A few thousand subjects in clinical trials are not enough to prove that the Hib vaccine causes less permanent disability or death than Hib in children who are breastfed exclusively for 13 weeks or more (Fig. 2).
There are not enough subjects in clinical trials to prove that the Hib vaccine poses less risk than Hib for children breastfed exclusively for 13 weeks or more.
How Accurate Are Epidemiological Studies of the Hib Vaccine?
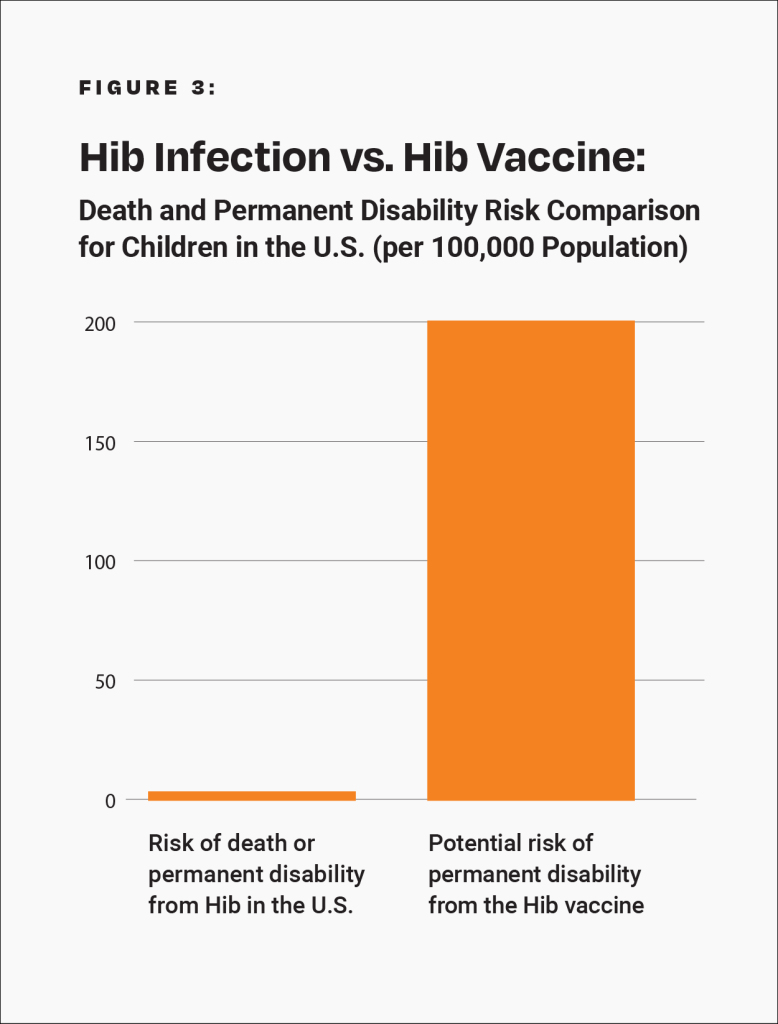
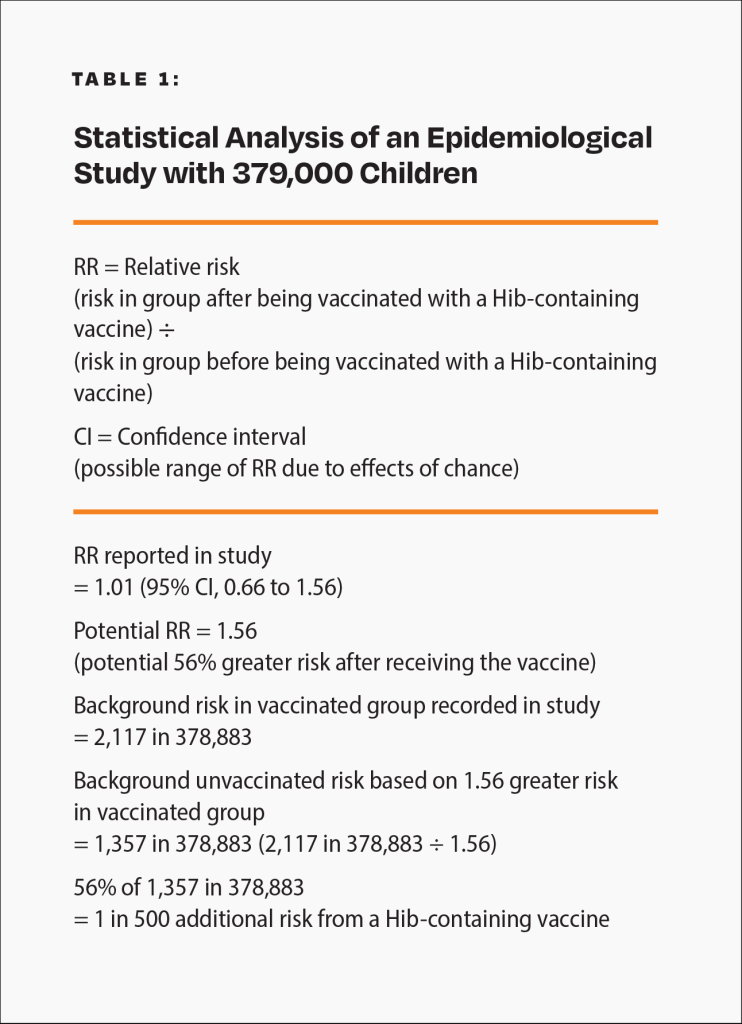
Epidemiological studies are hindered by the effects of chance. For example, a study published in the Journal of the American Medical Association (JAMA) involving about 379,000 children looked for an association between a Hib-containing vaccine and certain adverse events.19 Although the study found no association between the Hib-containing vaccine and the adverse events, it did not rule out the possibility that the vaccine increases the risk of an adverse event that leads to permanent disability by up to 56%. Consequently, the study did not rule out the possibility that such adverse events might occur up to 58 times more often than permanent disability from Hib in children who were breastfed exclusively for 13 weeks or more: 1 in 500 compared to 1 in 29,000 (Fig. 3 and Table 1). The range of possibilities found in the study makes the result inconclusive; even large epidemiological studies are not accurate enough to prove that the Hib vaccine causes less permanent disability or death than Hib in children who were breastfed exclusively for 13 weeks or more.
Is the Hib Vaccine Safer Than Hib?
It has not been proven that the Hib vaccine is safer than Hib. The vaccine package insert raises questions about safety testing for cancer, genetic mutations, and impaired fertility. Although VAERS tracks some adverse events, it is too inaccurate to measure against the risk of Hib. Clinical trials do not have the ability to detect less common adverse reactions, and epidemiological studies are limited by the effects of chance. Safety studies of the Hib vaccine are lacking in statistical power. A review of Hib vaccine safety studies conducted by the IOM found that the evidence was inadequate to rule out the possibility that Hib vaccination leads to transverse myelitis, Guillain-Barré syndrome, thrombocytopenia, and sudden infant death syndrome.11 In addition, studies have observed an association between the Hib vaccine and type 1 diabetes.10 Because permanent sequelae (aftereffects) from Hib are so rare (especially in children who were breastfed exclusively for 13 weeks or more), the level of accuracy of the research studies available is insufficient to prove that the Hib vaccine causes less permanent disability or death than Hib.
References
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, editors. Washington, D.C.: Public Health Foundation; 2021. 116. https://physiciansforinformedconsent.org/cdc-pink-book-14th-edition-2021/.
- Perdue DG, Bulkow LR, Gellin BG, Davidson M, Petersen KM, Singleton RJ, Parkinson AJ. Invasive Haemophilus influenzae disease in Alaskan residents aged 10 years and older before and after infant vaccination programs. JAMA. 2000 Jun 21;283(23):3089-94. https://pubmed.ncbi.nlm.nih.gov/10865303/.
- Langereis JD, de Jonge MI. Invasive disease caused by nontypeable Haemophilus influenzae. Emerg Infect Dis. 2015 Oct;21(10):1711-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4593434/.
- Merck. Rahway (NJ): Merck and Co., Inc. Liquid PedvaxHIB; revised 2023 Apr [cited 2024 May 19]. 1,6,7. https://www.merck.com/product/usa/pi_circulars/p/pedvax_hib/pedvax_pi.pdf.
- Sanofi Pasteur SA. Marcy L’Etoile, France: Sanofi Pasteur SA. ActHIB; revised 2022 Mar [cited 2024 May 19]. 6,7 https://www.fda.gov/media/74395/download.
- Between 2000 and 2004, the Vaccine Adverse Event Reporting System (VAERS) received 360 reports of seizure-related adverse events involving Hib vaccine in children <1 year of age.7 In the same time period, VAERS received 618 reports of seizure-related adverse events involving measles vaccine in children 1-2 years of age.8 This suggests that seizures from Hib vaccine may occur 58.3% as often as seizures from MMR vaccine, 58.3% of 1 in 6409 or about 1 in 1,098.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2024 April 3]. https://wonder.cdc.gov/vaers.html. Query for seizure-related events involving Haemophilus B conjugate vaccine, 2000-2004.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2022 October 20]. https://wonder.cdc.gov/vaers.html. Query for seizure-related events involving all measles-containing vaccines, 2000-2004.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004 Jul 21;292(3):356. https://jamanetwork.com/journals/jama/fullarticle/199117.
- Classen JB, Classen DC. Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after Hemophilus influenza B (HiB) immunization support causal relationship between immunization and IDDM. Autoimmunity. 2002 Jul;35(4):247. https://pubmed.ncbi.nlm.nih.gov/12482192/.
- Institute of Medicine (IOM). Adverse events associated with childhood vaccines: evidence bearing on causality. Washington, D.C.: National Academies Press; 1994. 243-63. https://pubmed.ncbi.nlm.nih.gov/25144097/.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Aluminum – vaccine risk statement (VRS). Aluminum in vaccines: what parents need to know; 2020 Aug; updated 2023 Apr [cited 2024 May 19]. https://physiciansforinformedconsent.org/aluminum-in-vaccines.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2024 April 9]. https://wonder.cdc.gov/vaers.html. Query for death and permanent disability involving Haemophilus B conjugate vaccine, 2000-2004.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Miller ER, Haber P, Hibbs B, Broder K. Chapter 21: surveillance for adverse events following immunization using the Vaccine Adverse Event Reporting System (VAERS). Atlanta: Centers for Disease Control and Prevention; 2011. 1,2,8. https://physiciansforinformedconsent.org/cdc-manual-for-the-surveillance-of-vaccine-preventable-diseases-5th-ed-chpt21-surv-adverse-events-2011.
- Centers for Disease Control and Prevention, Food and Drug Administration. Washington D.C.: U.S. Department of Health and Human Services. Guide to interpreting VAERS data; [cited 2022 May 28]. https://vaers.hhs.gov/data/dataguide.html.
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993 Jun 2;269(21):2765-8. https://www.sciencedirect.com/science/article/abs/pii/0163834394900515?via%3Dihub.
- Centers for Disease Control and Prevention. Washington D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2022 May 28]. https://wonder.cdc.gov/vaers.html.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4)Suppl:14. https://pubmed.ncbi.nlm.nih.gov/33003480/.
- Sun Y, Christensen J, Hviid A, Li J, Vedsted P, Olsen J, Vestergaard M. Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type b. JAMA. 2012 Feb 22;307(8):823-31. https://jamanetwork.com/journals/jama/fullarticle/1355993.
Published 2024 Aug