New Safety Concerns Raised About the Quantities of Aluminum in Childhood Vaccines
In 2011, Mitkus et al.1 published “Updated aluminum pharmacokinetics following infant exposures through diet and vaccination” in Vaccine, which updated an analysis published earlier by Keith et al.2 in 2002 that “analyzed the pharmacokinetics of aluminum for infant dietary and vaccine exposures,” and compared the resulting body burdens to those based on the minimal risk level (MRL) established by the ATSDR.3
In their analysis, Keith et al. used the MRL of 2 mg Al/kg/day for aluminum established by the ATSDR in 1999, based on Golub et al.’s 1989 aluminum lactate study. Since the ATSDR, at that time, did not account for the amount of aluminum absorbed by the gastrointestinal tract through aluminum lactate in its computations, Keith et al. relied on other studies that observed a 0.78% absorption from aluminum lactate and used that statistic in their calculations.
In 2008, the ATSDR lowered the MRL to 1 mg Al/kg/day for aluminum using a more recent aluminum lactate study. When computing the new MRL, the ATSDR included an additional modifying factor to “account for possible differences in the bioavailability of the aluminum lactate used in the Golub and Germann (2001) study and the bioavailability of aluminum from drinking water and a typical U.S. diet.” Additionally, in explaining the uncertainty factors used to derive the new MRL, the ATSDR cited studies that found “the bioavailability of aluminum from the typical U.S. diet was 0.1%.” Thus, an aluminum absorption of 0.1% was used to calculate the ATSDR’s 2008 MRL of 1 mg Al/kg/day.
Although Mitkus et al. included the ATSDR’s most recent MRL of 1 mg Al/kg/day in their computations, they used the aluminum absorption percentage of 0.78% from Keith et al.’s analysis instead of the 0.1% absorption that the 2008 ATSDR had accounted for in its computation of the new MRL. Consequently, the MRL curve calculated by Mitkus et al. is 7.8 times (0.78%/0.1%) greater than it would have been if they had used the aluminum absorption percentage that was used to calculate the ATSDR’s 2008 MRL for aluminum. Scaling down the MRL curve calculated by Mitkus et al. by a factor of 7.8 produces results that contradict the conclusions reached by Mitkus et al. concerning the safety of aluminum quantities in vaccines (Fig. 1).
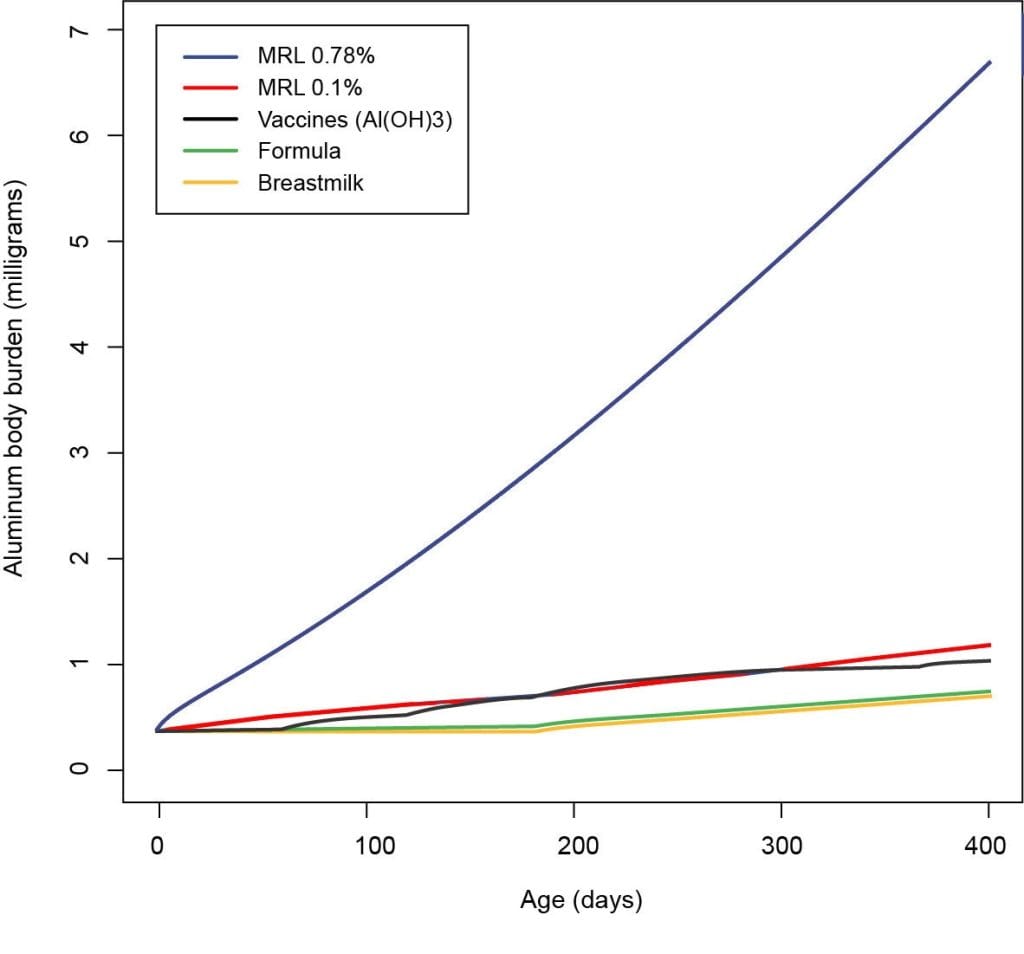
Figure 1: Body burden contributions of aluminum from diet and vaccines in infants, as shown in Mitkus et al. Figure 4, with MRL based on 0.78% aluminum absorption (blue) corrected to 0.1% aluminum absorption (red).
This graph shows the aluminum body burden (i.e., estimated amount of aluminum residing in the body) for infants calculated in the FDA paper Mitkus et al.: vaccines (black), formula (green) and breastmilk (yellow).
The MRL lines (blue and red) show the aluminum safety limit (i.e., estimated maximum amount of aluminum that an infant can safely handle).
The FDA paper Mitkus et al. assumed an incorrect aluminum absorption rate of 0.78% (blue). This level appears to be safe because it is significantly higher than the vaccine (black) line.
The corrected aluminum absorption rate is 0.1% (red). The corrected level raises concerns because it is close to the vaccine (black) line, indicating the aluminum amounts in vaccines may not be safe.
References
- Mitkus RJ, King DB, Hess MA, Forshee RA, Walderhaug MO. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. 2011 Nov 28;29(51):9538-43. https://www.ncbi.nlm.nih.gov/pubmed/22001122.
- Keith LS, Jones DE, Chou CH. Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine. 2002 May 31;20 Suppl 3:S13-7. https://www.ncbi.nlm.nih.gov/pubmed/12184359.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for aluminum. Washington, D.C.: U.S. Department of Health and Human Services; 2008. https://www.atsdr.cdc.gov/toxprofiles/tp22.pdf.
Published 2020 Mar; updated 2024 Oct
