MMR (Measles, Mumps and Rubella) – Vaccine Risk Statement (VRS)
MMR Vaccine (Measles, Mumps, and Rubella)
Is It Safer Than Measles, Mumps and Rubella?
What Is the MMR Vaccine?
The measles, mumps, and rubella (MMR) vaccine is a live virus vaccine that was introduced in 1963. It has significantly reduced the incidence of reported cases of measles, mumps, and rubella infections; however, vaccine immunity wanes over time.1-3
What Are Side Effect of the MMR Vaccine?
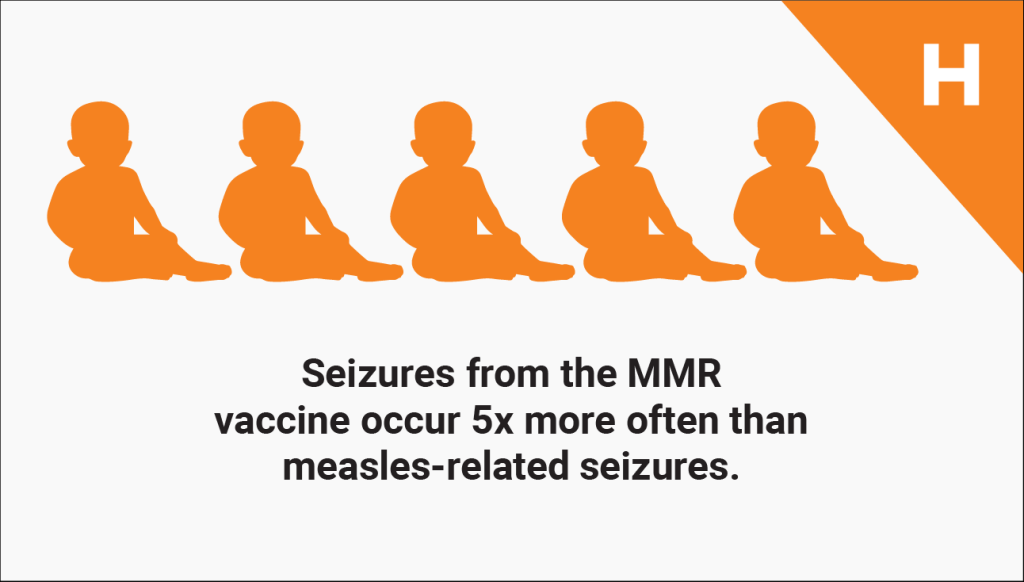
Common side effects of the MMR vaccine include fever, mild rash, and swelling of glands in the cheeks or neck.4 A more serious side effect is seizure, which occurs in about 1 in 640 children vaccinated with MMR5 — about five times more often than seizure from measles infection.6
Although severe potential side effects have been observed following MMR vaccination, including neurological disorders (e.g., encephalopathy, meningitis, ataxia, transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndrome, brachial neuritis, and hearing loss), autoimmune diseases (e.g., chronic arthritis), fibromyalgia, and chronic fatigue syndrome, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relationship between MMR vaccine” and those conditions.7 Additionally, the manufacturer’s package insert states, “M-M-R II vaccine has not been evaluated for carcinogenic or mutagenic potential or impairment of fertility.”8
How Are Risks of Vaccine Side Effects Measured?
Methods to measure vaccine risks include surveillance systems, clinical studies, and epidemiological studies.
How Accurate Is Surveillance of Adverse Events from the MMR Vaccine?
The government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 40 cases of death and permanent injury from the MMR vaccine are reported to VAERS annually.9 However, VAERS is a passive reporting system — authorities do not actively search for cases and do not actively remind doctors and the public to report cases. These limitations can lead to significant underreporting.10 The Centers for Disease Control and Prevention (CDC) states, “VAERS receives reports for only a small fraction of actual adverse events.”11 Indeed, as few as 1% of serious side effects from medical products are reported to passive surveillance systems,12 and as few as 1.6% of MMR-related seizures are reported to VAERS.13 In addition, VAERS reports are not proof that a side effect occurred, as the system is not designed to thoroughly investigate all cases.14 As a result, VAERS does not provide an accurate count of MMR vaccine side effects.
How Accurate Are Clinical Trials of the MMR Vaccine?
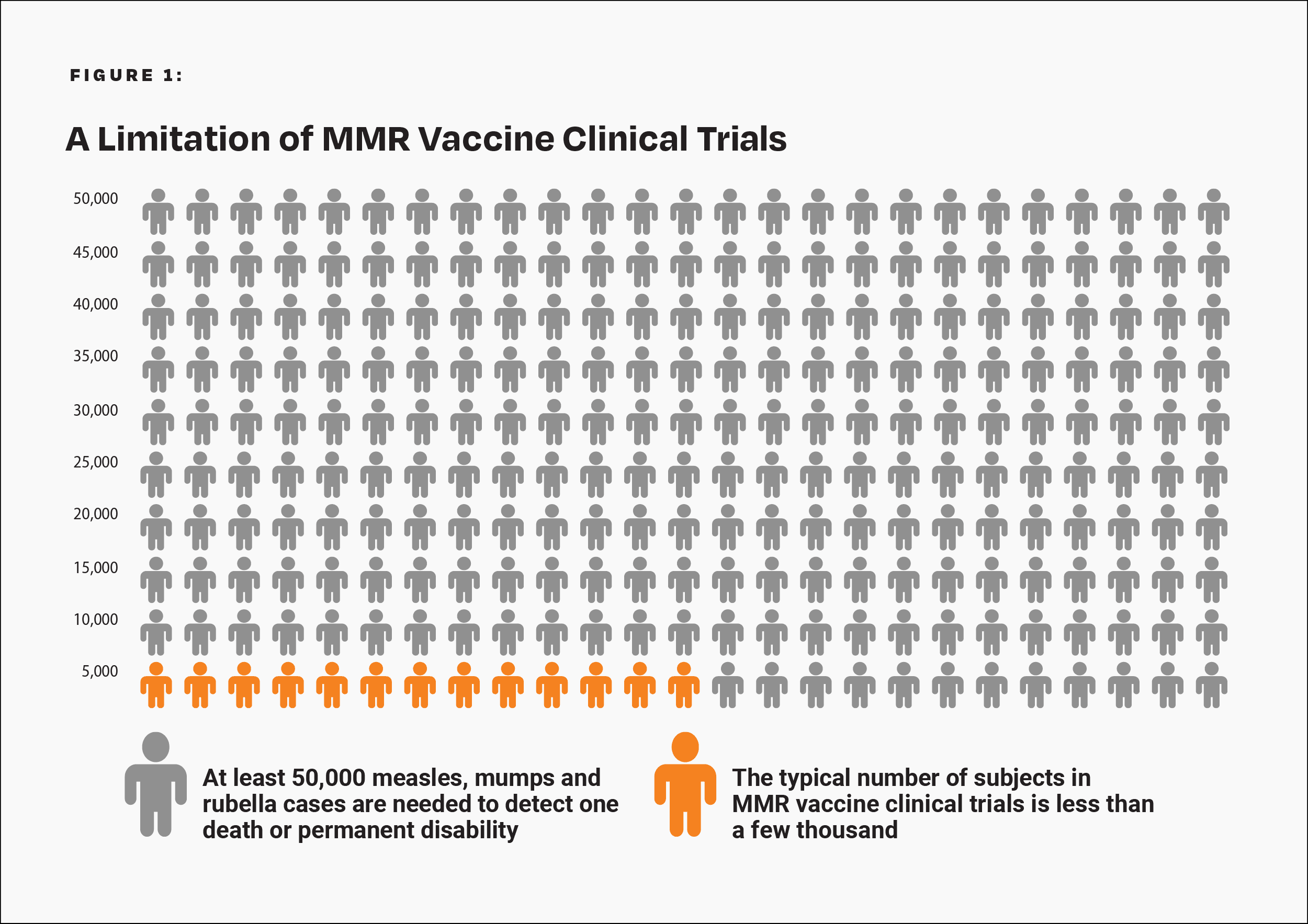
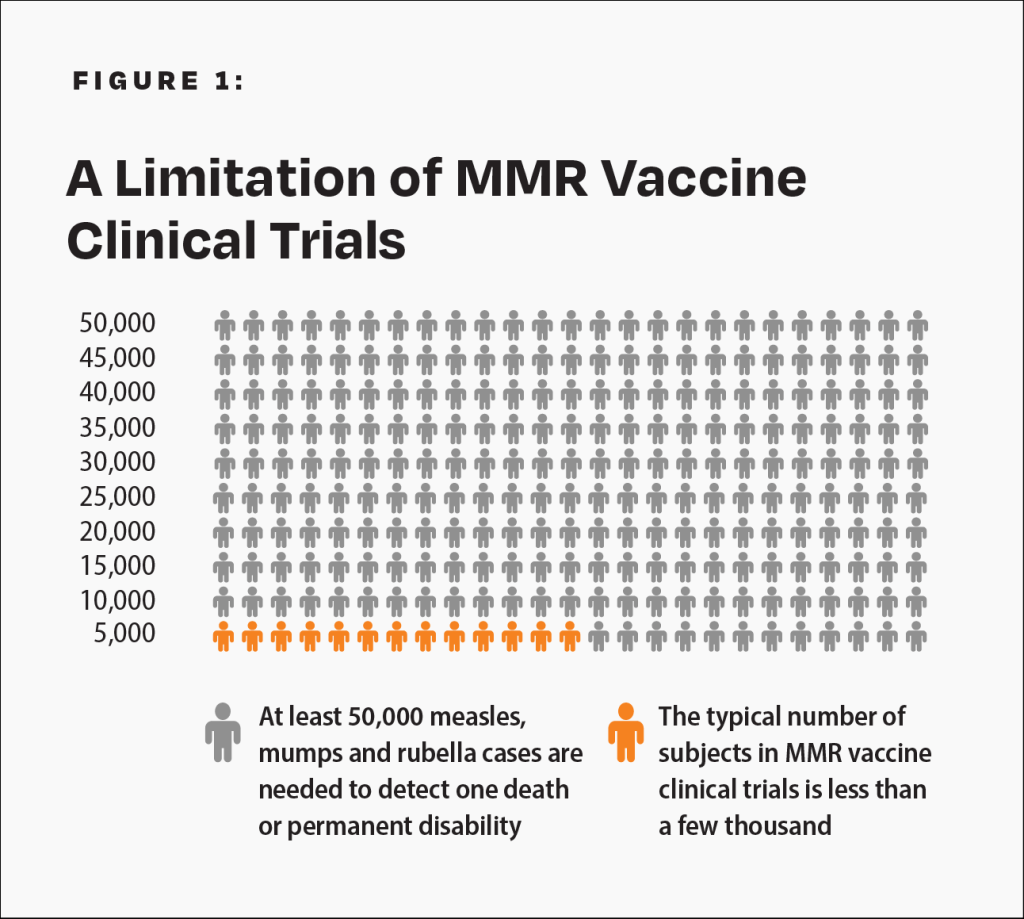
The CDC states, “Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.”10 For children under age 10 at normal risk (i.e., with normal levels of vitamin A and infected after birth), the pre-vaccine annual risk of death or permanent disability from measles, mumps, and rubella respectively was 1 in 1 million, 1 in 1.6 million, and 1 in 2.1 million.6,15-17 Therefore, the cumulative annual risk of a fatal or permanently disabling case of any of those diseases was about 1 in 500,000, and the risk over a 10-year span was 1 in 50,000. A few thousand subjects in clinical trials are not enough to prove that the MMR vaccine causes less permanent disability or death than measles, mumps, and rubella (Fig. 1). In addition, the lack of adequate clinical trials of the MMR vaccine resulted in the manufacturer’s package insert data to be reliant on passive surveillance for rates of MMR-related neurological adverse reactions, permanent disability, and death.8
How Accurate Are Epidemiological Studies of the MMR Vaccine?
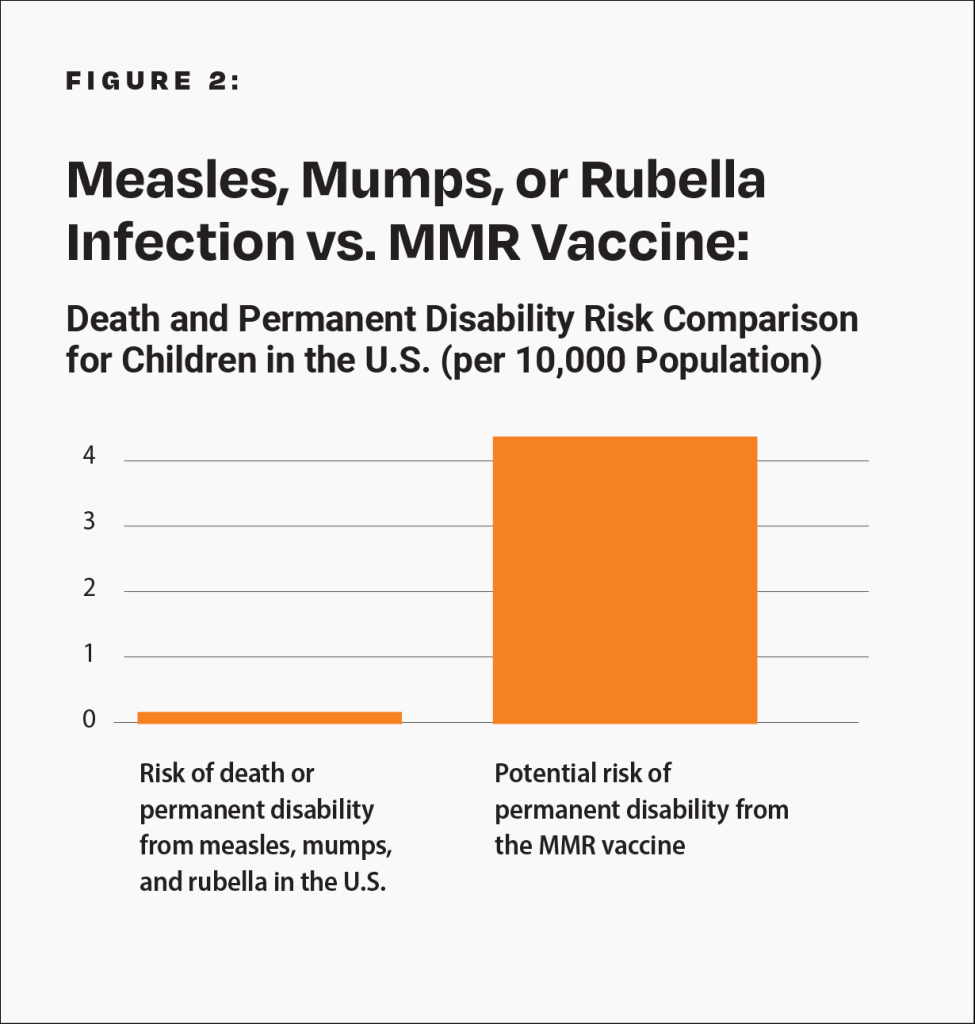
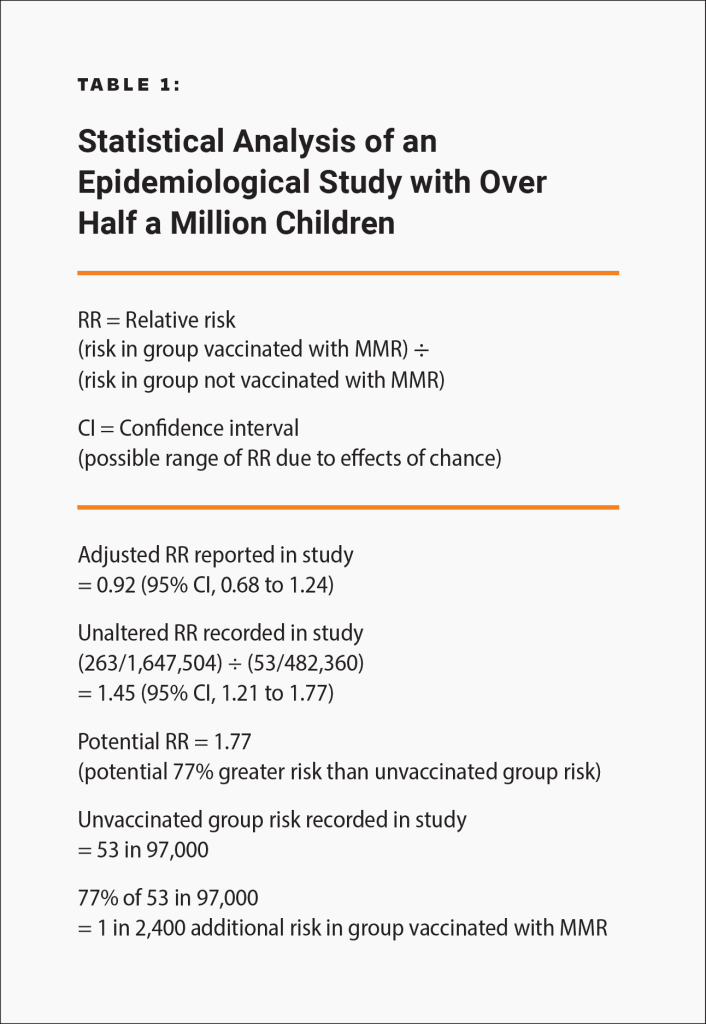
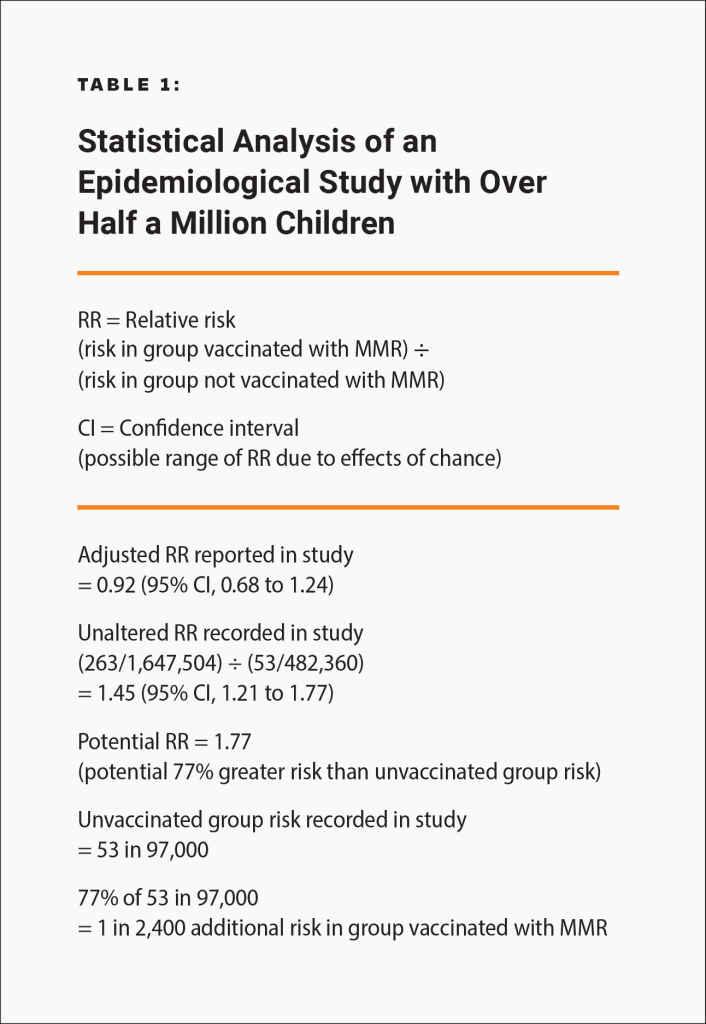
Epidemiological studies are hindered by the effects of chance and possible confounders — additional factors that could conceivably affect the groups being studied. For example, there is a well-known 2002 Danish study published in the New England Journal of Medicine involving about 537,000 children that looked for an association between the MMR vaccine and certain adverse events.18 The raw data in the study was adjusted, in an attempt to account for potential confounders, and the study found no association between the MMR vaccine and the adverse events. However, because there is no evidence that the estimated confounders used to adjust the raw data were actually confounders, the study did not rule out the possibility that the MMR vaccine increases the risk of an adverse event that leads to permanent injury by up to 77%. Consequently, the study did not rule out the possibility that such adverse events might occur up to 21 times more often than death or permanent disability from measles, mumps, and rubella in children at normal risk (i.e., with normal levels of vitamin A and infected after birth): 1 in 2,400 compared to 1 in 50,000 (Fig. 2 and Table 1). The range of possibilities found in the study, between the adjusted data and the raw data, makes the result inconclusive; even large epidemiological studies are not accurate enough to prove that the MMR vaccine causes less death or permanent injury than measles, mumps, and rubella.
Is the MMR Vaccine Safer Than Measles, Mumps, and Rubella?
It has not been proven that the MMR vaccine is safer than measles, mumps, and rubella. The vaccine package insert raises questions about safety testing for cancer, genetic mutations, and impaired fertility. Although VAERS tracks some adverse events, it is too inaccurate to measure against the risk of measles, mumps, and rubella. Clinical trials do not have the ability to detect less common adverse reactions, and epidemiological studies are limited by the effects of chance and possible confounders. Safety studies of the MMR vaccine are particularly lacking in statistical power. A review of more than 60 MMR vaccine studies conducted for the Cochrane Library states, “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”19 Because permanent sequelae (aftereffects) from measles, mumps, and rubella are so rare (especially in children with normal levels of vitamin A and infected after birth),6,15-17 the level of accuracy of the research studies available is insufficient to rule out the possibility that the MMR vaccine causes greater death or permanent disability than measles, mumps, and rubella.
A 2002 Danish study did not rule out the possibility that the MMR vaccine can cause an adverse event leading to permanent disability 21 times more often than measles, mumps, and rubella can be fatal or lead to permanent disability for U.S. children at normal risk (i.e., with normal levels of vitamin A and infected after birth).
Although severe potential side effects have been observed following MMR vaccination, including neurological disorders (e.g., encephalopathy, meningitis, ataxia, transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndrome, brachial neuritis, and hearing loss), autoimmune diseases (e.g., chronic arthritis), fibromyalgia, and chronic fatigue syndrome, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relationship between MMR vaccine” and those conditions.7 Additionally, the manufacturer’s package insert states, “M-M-R II vaccine has not been evaluated for carcinogenic or mutagenic potential or impairment of fertility.”8

How Are Risks of Vaccine Side Effects Measured?
Methods to measure vaccine risks include surveillance systems, clinical studies, and epidemiological studies.
How Accurate Is Surveillance of Adverse Events from the MMR Vaccine?
The government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 40 cases of death and permanent injury from the MMR vaccine are reported to VAERS annually.9 However, VAERS is a passive reporting system — authorities do not actively search for cases and do not actively remind doctors and the public to report cases. These limitations can lead to significant underreporting.10 The Centers for Disease Control and Prevention (CDC) states, “VAERS receives reports for only a small fraction of actual adverse events.”11 Indeed, as few as 1% of serious side effects from medical products are reported to passive surveillance systems,12 and as few as 1.6% of MMR-related seizures are reported to VAERS.13 In addition, VAERS reports are not proof that a side effect occurred, as the system is not designed to thoroughly investigate all cases.14 As a result, VAERS does not provide an accurate count of MMR vaccine side effects.
How Accurate Are Clinical Trials of the MMR Vaccine?
The CDC states, “Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.”10 For children under age 10 at normal risk (i.e., with normal levels of vitamin A and infected after birth), the pre-vaccine annual risk of death or permanent disability from measles, mumps, and rubella respectively was 1 in 1 million, 1 in 1.6 million, and 1 in 2.1 million.6,15-17 Therefore, the cumulative annual risk of a fatal or permanently disabling case of any of those diseases was about 1 in 500,000, and the risk over a 10-year span was 1 in 50,000. A few thousand subjects in clinical trials are not enough to prove that the MMR vaccine causes less permanent disability or death than measles, mumps, and rubella (Fig. 1). In addition, the lack of adequate clinical trials of the MMR vaccine resulted in the manufacturer’s package insert data to be reliant on passive surveillance for rates of MMR-related neurological adverse reactions, permanent disability, and death.8
There are not enough subjects in clinical trials to prove that the MMR vaccine poses less risk than measles, mumps, and rubella.
How Accurate Are Epidemiological Studies of the MMR Vaccine?
Epidemiological studies are hindered by the effects of chance and possible confounders — additional factors that could conceivably affect the groups being studied. For example, there is a well-known 2002 Danish study published in the New England Journal of Medicine involving about 537,000 children that looked for an association between the MMR vaccine and certain adverse events.18 The raw data in the study was adjusted, in an attempt to account for potential confounders, and the study found no association between the MMR vaccine and the adverse events. However, because there is no evidence that the estimated confounders used to adjust the raw data were actually confounders, the study did not rule out the possibility that the MMR vaccine increases the risk of an adverse event that leads to permanent injury by up to 77%. Consequently, the study did not rule out the possibility that such adverse events might occur up to 21 times more often than death or permanent disability from measles, mumps, and rubella in children at normal risk (i.e., with normal levels of vitamin A and infected after birth): 1 in 2,400 compared to 1 in 50,000 (Fig. 2 and Table 1). The range of possibilities found in the study, between the adjusted data and the raw data, makes the result inconclusive; even large epidemiological studies are not accurate enough to prove that the MMR vaccine causes less death or permanent injury than measles, mumps, and rubella.
Is the MMR Vaccine Safer Than Measles, Mumps, and Rubella?
It has not been proven that the MMR vaccine is safer than measles, mumps, and rubella. The vaccine package insert raises questions about safety testing for cancer, genetic mutations, and impaired fertility. Although VAERS tracks some adverse events, it is too inaccurate to measure against the risk of measles, mumps, and rubella. Clinical trials do not have the ability to detect less common adverse reactions, and epidemiological studies are limited by the effects of chance and possible confounders. Safety studies of the MMR vaccine are particularly lacking in statistical power. A review of more than 60 MMR vaccine studies conducted for the Cochrane Library states, “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”19 Because permanent sequelae (aftereffects) from measles, mumps, and rubella are so rare (especially in children with normal levels of vitamin A and infected after birth),6,15-17 the level of accuracy of the research studies available is insufficient to rule out the possibility that the MMR vaccine causes greater death or permanent disability than measles, mumps, and rubella.
A 2002 Danish study did not rule out the possibility that the MMR vaccine can cause an adverse event leading to permanent disability 21 times more often than measles, mumps, and rubella can be fatal or lead to permanent disability for U.S. children at normal risk (i.e., with normal levels of vitamin A and infected after birth).
References
- LeBaron CW, Beeler J, Sullivan BJ, Forghani B, Bi D, Beck C, Audet S, Gargiullo P. Persistence of measles antibodies after 2 doses of measles vaccine in a postelimination environment. Arch Pediatr Adolesc Med. 2007 Mar;161(3):294-301. https://pubmed.ncbi.nlm.nih.gov/17339511/.
- Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. 2018 Mar 21;10(433):2. http://stm.sciencemag.org/content/10/433/eaao5945.
- Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. 2008 Apr 1;197(7):955. https://pubmed.ncbi.nlm.nih.gov/18419470/.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Vaccines and immunizations: possible side effects from vaccines; [cited 2023 Dec 28]. https://physiciansforinformedconsent.org/cdc-vaccines-and-immunizations-possible-side-effects-from-vaccines/.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004 Jul 21;292(3):356. https://jamanetwork.com/journals/jama/fullarticle/199117.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Measles – disease information statement (DIS). 2017 Oct; updated 2024 Aug. https://physiciansforinformedconsent.org/measles.
- Institute of Medicine (IOM). Adverse effects of vaccines: evidence and causality. Washington, D.C.: National Academies Press; 2012. 119-217. https://www.ncbi.nlm.nih.gov/books/NBK190024/pdf/Bookshelf_NBK190024.pdf.
- Merck. Rahway (NJ): Merck and Co., Inc. M-M-R II (measles, mumps, and rubella virus vaccine live); revised 2023 Oct [cited 2024 Jan 27]. 8. https://www.merck.com/product/usa/pi_circulars/m/mmr_ii/mmr_ii_pi.pdf.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2024 Feb 12]. https://wonder.cdc.gov/vaers.html. Query for death and permanent disability involving all measles-containing vaccines, 2011-2015.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Miller ER, Haber P, Hibbs B, Broder Chapter 21: surveillance for adverse events following immunization using the Vaccine Adverse Event Reporting System (VAERS). Atlanta: Centers for Disease Control and Prevention; 2011. 1,2,8. https://physiciansforinformedconsent.org/cdc-manual-for-the-surveillance-of-vaccine-preventable-diseases-5th-ed-chpt21-surv-adverse-events-2011.
- Centers for Disease Control and Prevention, Food and Drug Administration. Washington, D.C.: U.S. Department of Health and Human Services. Guide to interpreting VAERS data; [cited 2022 May 28]. https://vaers.hhs.gov/data/dataguide.html.
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993 Jun 2;269(21):2765- https://www.sciencedirect.com/science/article/abs/pii/0163834394900515?via%3Dihub.
- Doshi P. The unofficial vaccine educators: are CDC funded non-profits sufficiently independent? [letter]. BMJ. 2017 Nov 7 [cited 2017 Nov 20];359:j5104. http://www.bmj.com/content/359/bmj. j5104/rr-13.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2022 May 28]. https://wonder.cdc.gov/vaers.html.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4):4. https://pubmed.ncbi.nlm.nih.gov/33003480/.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Mumps – disease information statement (DIS). Mumps: what parents need to know. 2024 Aug. https://physiciansforinformedconsent.org/mumps.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Rubella – disease information statement (DIS). Rubella: what parents need to know. 2024 Aug. https://physiciansforinformedconsent.org/rubella.
- Madsen KM, Hviid A, Vestergaard M, Schendel D, WohlFahrt J, Thorsen P, Olsen J, Melbye M. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002 Nov 7;347(19):1477,1480. https://www.nejm.org/doi/full/10.1056/NEJMoa021134?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed.
- Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database of Syst Rev. 2012 Feb 15;(2). https://pubmed.ncbi.nlm.nih.gov/22336803/.
Published 2024 Aug; updated 2024 Dec