This vaccine has not been approved or licensed, and is still under investigation.1
Moderna COVID-19 Vaccine:
Short-Term Efficacy & Safety Data
 1. WHAT IS THE MODERNA COVID-19 VACCINE?
1. WHAT IS THE MODERNA COVID-19 VACCINE?
The Moderna COVID-19 vaccine (mRNA-1273) is made from synthetic genetic material that is immersed in fatty substances, including cholesterol and polyethylene glycol (PEG). More specifically, modified RNA molecules that encode for a mutated spike (S) protein antigen of the SARS-CoV-2 virus, the virus that can cause COVID-19, are immersed in lipid nanoparticles. The drug is administered in two intramuscular 100 mcg doses, 28 days apart.1
 2. HOW WAS THE VACCINE STUDIED PRIOR TO OBTAINING EUA?
2. HOW WAS THE VACCINE STUDIED PRIOR TO OBTAINING EUA?
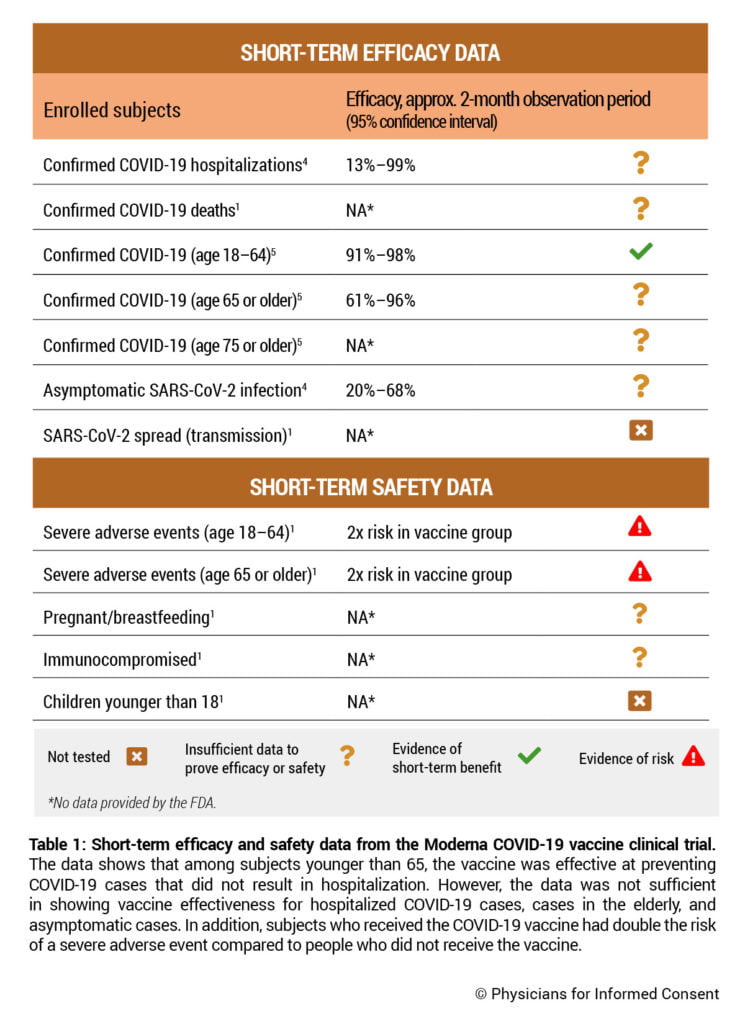
The Moderna COVID-19 vaccine obtained emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) on Dec. 18, 2020, and is currently investigational.2 The vaccine was studied through nonclinical data from rats, mice, hamsters, and nonhuman primates, and clinical data from humans.3 The EUA was based on a human clinical trial comparing approximately 15,000 subjects who received the vaccine with 15,000 subjects who did not receive the vaccine (Table 1).1 The trial included a median observation period of nine weeks; 53.6% of subjects were followed up for about two months after the second dose.1 The FDA states that due to the length of the clinical trial’s observation period, “it is not possible to assess sustained efficacy over a period longer than 2 months.”1
 3. DOES THE VACCINE PREVENT HOSPITALIZATIONS AND DEATHS?
3. DOES THE VACCINE PREVENT HOSPITALIZATIONS AND DEATHS?
Since only 10 hospitalized cases and one death of COVID-19 were observed, the clinical trial did not have enough statistical power to accurately measure the vaccine’s ability to prevent hospitalizations or deaths from COVID-19.1,4 The vaccine may be only 13% effective against hospitalized COVID-19 cases. See Table 1. The FDA states, “A larger number of individuals at high risk of COVID-19 and higher attack rates would be needed to confirm efficacy of the vaccine against mortality.”1
 4. HOW EFFECTIVE IS THE VACCINE IN ADULTS AND THE ELDERLY?
4. HOW EFFECTIVE IS THE VACCINE IN ADULTS AND THE ELDERLY?
Vaccine effectiveness was calculated by observing the vaccine status of 196 COVID-19 cases, where a COVID-19 case was defined as a positive SARS-CoV-2 test together with the presence of at least either one COVID-19 respiratory symptom or two non-respiratory symptoms, at least 14 days after the second dose. In subjects 18 to 64 years old, the vaccine was 91%–98% effective over a two-month observation period.8 However, since there were only 33 COVID-19 cases observed in subjects 65 years or older, the clinical trial did not have enough statistical power to accurately measure the vaccine’s effectiveness in that age group. The vaccine may be only 61% effective in subjects 65 years or older and 0% effective in subjects 75 years or older.5 See Table 1. Subjects 65 years or older comprise about 80% of all COVID-19 deaths, and subjects 75 years or older comprise about 60% of all COVID-19 deaths.6
 5. IS THE VACCINE EFFECTIVE IN CHILDREN?
5. IS THE VACCINE EFFECTIVE IN CHILDREN?
Safety and efficacy data was not collected for children younger than 18 years old.1 See Table 1.
 6. IS THE VACCINE EFFECTIVE IN PREVENTING INFECTION WITH SARS-COV-2 OR THE SPREAD OF COVID-19?
6. IS THE VACCINE EFFECTIVE IN PREVENTING INFECTION WITH SARS-COV-2 OR THE SPREAD OF COVID-19?
The Moderna clinical trial was not designed to observe asymptomatic infection with SARS-CoV-2 or the effect of the vaccine on the spread (transmission) of COVID-19. Consequently, the FDA states that “it is possible that asymptomatic infections may not be prevented as effectively as symptomatic infections” and “data are limited to assess the effect of the vaccine against transmission of SARS-CoV-2 from individuals who are infected despite vaccination.” Furthermore, “additional evaluations including data from clinical trials and from vaccine use post-authorization will be needed to assess the effect of the vaccine in preventing virus shedding and transmission, in particular in individuals with asymptomatic infection.”1 Approximately 40% of SARS-CoV-2 infections are asymptomatic.7
To try to address the limitations above, Moderna performed an analysis of a few cases that tested positive but reported no symptoms. However, the analysis still lacked statistical power to produce an accurate measurement, and the vaccine may be only 20% effective in preventing asymptomatic cases.4
 7. WHAT IS THE RISK OF A SEVERE SIDE EFFECT FROM THE VACCINE?
7. WHAT IS THE RISK OF A SEVERE SIDE EFFECT FROM THE VACCINE?
The Moderna COVID-19 vaccine clinical trial found the overall incidence of severe adverse events during the two-month observation period to be 2% or 1 in 50 in vaccinated subjects between 18 and 64 years old and 1.2% in the unvaccinated group, resulting in a vaccine risk of 0.8% or 1 in 125 vaccinated subjects. The incidence of severe adverse events was 1.7% or 1 in 59 in vaccinated subjects 65 years or older and 0.8% in the unvaccinated group, resulting in a vaccine risk of 0.9% or 1 in 111 vaccinated subjects.1 Consequently, subjects who received the vaccine had nearly double the risk of a severe adverse event occurring in the two-month observation period compared to subjects who did not receive the vaccine. See Table 1. A severe adverse event was one that persisted for longer than a week and either prevented the ability to perform daily activities and required medical intervention, or required hospitalization.1,8
Additionally, as there were only 7,500 subjects 18 to 53 years of age who received the vaccine,1 and since as of March 23, 2021, about 1 in 13,000 people 18 to 39 years of age contracted a fatal case of COVID-19 in the U.S.,6 the clinical trial does not have sufficient data to determine safety in subjects who are 18 to 39 years of age. Per the FDA, “There are currently insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 18 years of age, pregnant and lactating individuals, and immunocompromised individuals.”1 And, because all subjects were observed for only two months, the long-term safety of the vaccine for any age group is not known. The FDA states, “Long-term safety and long-term effectiveness are areas the Sponsor [Moderna] identified as missing information.”1
 8. IS THE COVID-19 VACCINE EFFECTIVE AND SAFER THAN COVID-19?
8. IS THE COVID-19 VACCINE EFFECTIVE AND SAFER THAN COVID-19?
The extent to which the Moderna COVID-19 vaccine is effective and safer than COVID-19 is not known. The clinical trial indicates that in subjects 65 years or older, the vaccine may be only 61% effective, and in subjects 75 years or older, the age group that comprises about 60% of all COVID-19 deaths, the vaccine may be 0% effective. The clinical trial did not have enough statistical power to measure the vaccine’s ability to prevent hospitalizations and deaths, and the trial had limited data to assess whether the vaccine prevents asymptomatic infection or spread (transmission) of the virus.
Severe adverse events in the vaccine group occurred in 1 in 50 subjects between 18 and 64 years old and in 1 in 59 subjects 65 years or older in the Moderna clinical trial. Those subjects were unable to perform normal daily activities for more than seven days and required medical attention. Furthermore, for people 18 to 39 years of age, the clinical trial did not include enough subjects to be able to show that the vaccine is safer than the disease, and because the clinical trial observation period lasted only two months, the incidence of long-term side effects from the vaccine for any age group is not known.
REFERENCES
- U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Moderna COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 17, 2020: 5, 13, 17, 21, 24, 29, 30, 36-38, 46-49. https://www.fda.gov/media/144434/download.
- Hinton, Denise M. (U.S. Food and Drug Administration). Letter to: Carlota Vinals (ModernaTX, Inc.). 2021 Feb 25. https://www.fda.gov/media/144636/download.
- ModernaTX, Inc. MRNA-1273 sponsor briefing document: Vaccines and Related Biological Products Advisory Committee; meeting date: 17 December 2020. https://www.fda.gov/media/144452/download.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine; [cited 2021 Mar 24]. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-moderna-vaccine.html.
- U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Moderna COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 17, 2020. Table 17: final scheduled efficacy analysis, primary endpoint, COVID-19 starting 14 days after the second dose per adjudication committee assessments, per-protocol set; 29. https://www.fda.gov/media/144434/download.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Weekly updates by select demographic and geographic characteristics: provisional death counts for coronavirus disease (COVID-19); [cited 2021 Mar 23]. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#AgeAndSex.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. COVID-19 pandemic planning scenarios; [updated 2020 Sep 10; cited 2021 Jan 13]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html.
- ModernaTX, Inc. A phase 3, randomized, stratified, observer-blind, placebo-controlled study to evaluate the efficacy, safety, and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine in adults aged 18 years and older; protocol mRNA-1273-P301, amendment 6. 2020 Dec 23. https://www.modernatx.com/sites/default/files/content_documents/Final%20mRNA-1273-P301%20Protocol%20Amendment%206%20-%2023Dec2020.pdf
These statements are intended for informational purposes only and should not be construed as personal medical advice.
© 2021 Physicians for Informed Consent, an independent 501(c)(3) nonprofit educational organization. All rights reserved. Apr 2021.