This vaccine has not been approved or licensed for children 15 years or younger.1
Pfizer COVID-19 Vaccine:
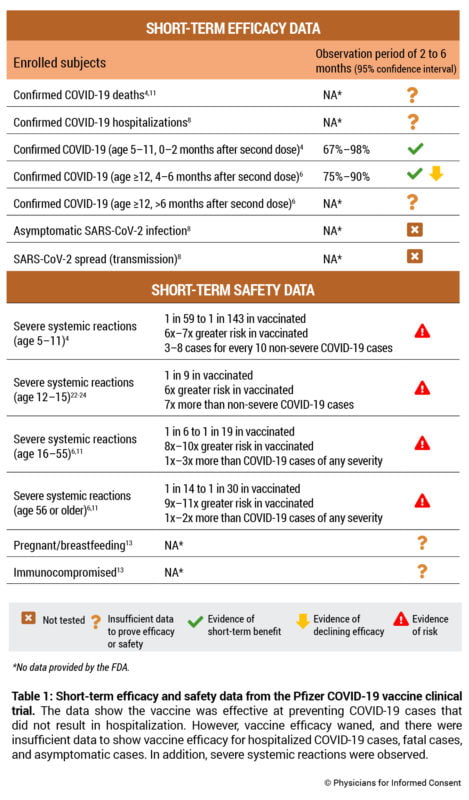
Short-Term Efficacy & Safety Data
 1. WHAT IS THE PFIZER COVID-19 VACCINE?
1. WHAT IS THE PFIZER COVID-19 VACCINE?
The Pfizer COVID-19 vaccine is made from synthetic genetic material that is immersed in fatty substances, including cholesterol and polyethylene glycol (PEG). More specifically, modified RNA molecules that encode for a mutated spike (S) protein antigen of the Wuhan-Hu-1 SARS-CoV-2 strain, the original virus that caused COVID-19, are immersed in lipid nanoparticles. The drug is administered in two intramuscular doses (30 mcg each for individuals aged 12 or older; 10 mcg each for children aged 5 to 11), 21 days apart.2-4
 2. HOW WAS THE VACCINE STUDIED PRIOR TO OBTAINING FDA APPROVAL?
2. HOW WAS THE VACCINE STUDIED PRIOR TO OBTAINING FDA APPROVAL?
The Pfizer COVID-19 vaccine was studied through nonclinical data from rats and nonhuman primates, and a human clinical trial comparing approximately 22,000 subjects who received the vaccine with 22,000 subjects who did not receive the vaccine (Table 1).5 About 42% of the subjects in the trial were observed for less than four months after the second dose and 51% were observed for four to less than six months after the second dose.6
Five times more vaccinated subjects were excluded from the clinical trial compared to subjects who did not receive the vaccine (311 compared to 60). The reason for those exclusions provided by the U.S. Food and Drug Administration (FDA) is “other important protocol deviations.” More specific reasons were not provided.7
 3. DOES THE VACCINE PREVENT HOSPITALIZATIONS AND DEATHS?
3. DOES THE VACCINE PREVENT HOSPITALIZATIONS AND DEATHS?
Since the number of hospitalized cases of COVID-19 have not been published in clinical trial documents, the vaccine’s ability to prevent hospitalizations from COVID-19 is unclear.6 See Table 1. Regarding deaths, the FDA states, “A larger number of individuals at high risk of COVID-19 and higher attack rates would be needed to confirm efficacy of the vaccine against mortality.”8
Of note, a study of a COVID-19 outbreak in July 2021 published in Eurosurveillance observed that 100% of severe, critical, and fatal cases of COVID-19 occurred in vaccinated individuals.9 A Centers for Disease Control and Prevention (CDC) study of another COVID-19 outbreak in July 2021 observed similar data and found that 80% of hospitalized cases were fully vaccinated.10
 4. HOW EFFECTIVE IS THE VACCINE IN ADULTS?
4. HOW EFFECTIVE IS THE VACCINE IN ADULTS?
Vaccine efficacy was calculated by observing the vaccination status of 971 COVID-19 cases (including Alpha and Beta variants of concern11), where a COVID-19 case was defined as the presence of at least one COVID-19 symptom and a positive SARS-CoV-2 test at least seven days after the second dose. In subjects 12 years or older, the vaccine was 93%–98% effective within two months of the second dose. However, between two and four months after the second dose, vaccine efficacy declined to 87%–93%, and between four and six months after the second dose, vaccine efficacy declined to 75%–90%.6
 5. IS THE VACCINE EFFECTIVE IN CHILDREN?
5. IS THE VACCINE EFFECTIVE IN CHILDREN?
The vaccine was authorized for use in children 12 to 15 years of age in May 2021, “although limited scientific information is available,” per Pfizer,12 and was 93%–98% effective over a two-month observation period.6 The vaccine was authorized for use in children 5 to 11 years of age in October 2021 and was 67%–98% effective over a two-month observation period.4
In the clinical trial, there were zero cases of severe COVID-19 in children who did not receive the vaccine. For adolescents 12 to 15 years of age, the FDA states, “There were no reports of severe COVID-19 cases.”13 For children 5 to 11 years of age, the FDA states, “None of these cases met the criteria for severe infection.”4 As such, the ability of the vaccine to prevent hospitalizations or deaths from COVID-19 in children 5 to 15 years of age is not known.
 6. WHAT ABOUT A BOOSTER DOSE?
6. WHAT ABOUT A BOOSTER DOSE?
Between September 2021 and January 2022, the FDA granted emergency use authorization (EUA) for a booster dose of the Pfizer COVID-19 vaccine for individuals 12 years of age or older.14,15 However, the clinical trial states, “Efficacy was not evaluated for Phase 3 BNT162b2 booster group participants,” and vaccine efficacy was inferred based on antibody levels observed in about 300 vaccinated subjects over a one-month time period.16
 7. DOES THE VACCINE PREVENT INFECTION OR TRANSMISSION?
7. DOES THE VACCINE PREVENT INFECTION OR TRANSMISSION?
The Pfizer clinical trial was not designed to observe asymptomatic infection with SARS-CoV-2 or the effect of the vaccine on the spread (transmission) of COVID-19. Consequently, the FDA states that “it is possible that asymptomatic infections may not be prevented as effectively as symptomatic infections” and “data are limited to assess the effect of the vaccine against transmission of SARS-CoV-2 from individuals who are infected despite vaccination.”8 Approximately 33% of SARS-CoV-2 infections are asymptomatic.17,18
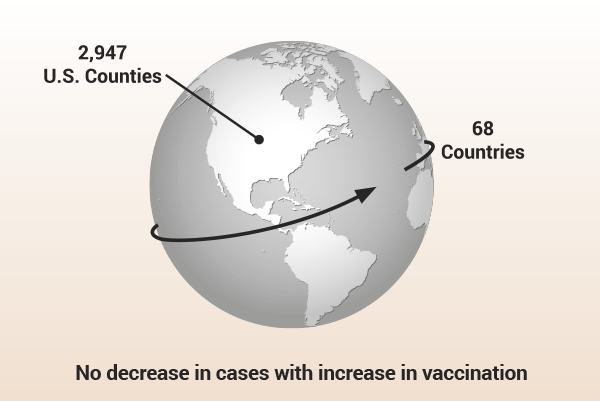
Of note, a study of a COVID-19 outbreak in July 2021 published in Eurosurveillance9 found that “all transmissions between patients and staff occurred between masked and vaccinated individuals, as experienced in an outbreak from Finland.” The authors stated that the study “challenges the assumption that high universal vaccination rates will lead to herd immunity and prevent COVID-19 outbreaks.” A CDC study of another COVID-19 outbreak in July 2021 observed similar data and found that 74% of cases were fully vaccinated.10 A Harvard study investigating COVID-19 cases across 68 countries and across 2,947 counties in the U.S. found “there also appears to be no significant signaling of COVID-19 cases decreasing with higher percentages of population fully vaccinated.”19
 8. WHAT IS THE RISK OF A SEVERE SIDE EFFECT FROM THE VACCINE?
8. WHAT IS THE RISK OF A SEVERE SIDE EFFECT FROM THE VACCINE?
The Pfizer COVID-19 vaccine clinical trial found that the vaccine causes severe (grade 3) systemic reactions, which include fever greater than 102.1° F; vomiting that requires IV hydration; diarrhea of six or more loose stools in 24 hours; and severe fatigue, severe headache, severe muscle pain, or severe joint pain that prevents daily activity.11,20 Trial data21 demonstrate that severe systemic reactions occur in 1 in 6 to 1 in 143 vaccinated subjects. See Table 1 and Supplement 1.4,6,11,22-24 The clinical trial also found that 1 in about 1,100 vaccinated children 12 to 15 years of age had a grade 4 systemic reaction (fever greater than 104.0° F) after the first dose that required an emergency room (ER) visit and withdrawal from the study.22

Of note, approximately 3,400 or 8% of subjects 16 years or older experienced “suspected COVID-19” because they had symptoms but were not confirmed by testing for SARS-CoV-2; two of these cases required hospitalization, both of which were in the vaccinated group. These cases could represent other influenza-like illness and adverse events; 409 such cases occurred in the vaccinated group within seven days of injection whereas 287 such cases occurred in the unvaccinated group in the same time period. Only the cases that were reported as serious were recorded as adverse events.8 In the clinical trial, only 5% of all illnesses suspected of being COVID-19 were actually found to be COVID-19.
After mass vaccination with the Pfizer COVID-19 vaccine began, the CDC recorded about 5,000 “health impact events” among 215,000 vaccinated subjects (1 in 43) that, similar to the definition of severe systemic reactions in the clinical trial, prevented the ability to perform normal daily activities, including work, and required medical attention.25
Concerningly, post-EUA safety surveillance reports have identified serious risks for myocarditis and pericarditis in subjects under age 40, within seven days of vaccination. In boys aged 16 or 17, after the second dose of the Pfizer COVID-19 vaccine, the risk of myocarditis or pericarditis is 1 in 5,000.16 Additionally, since the clinical trial observed only about 1,100 vaccinated children aged 12 to 1512 and 1,500 vaccinated children aged 5 to 11,4 there were not enough children included in the trial to be able to show the vaccine is safer than the disease. As of Nov. 3, 2021, the chance of a child 17 years or younger contracting SARS-CoV-2 and dying from COVID-19 was
1 in 126,000 or 0.0008%.26
Moreover, per the FDA, there are currently insufficient data to make conclusions about the safety of the vaccine in subpopulations such as pregnant and lactating individuals, and immunocompromised individuals.13 Per Pfizer, the vaccine “has not been evaluated for the potential to cause carcinogenicity, genotoxicity, or impairment of male fertility.”11 And, because all subjects were observed for only two to six months, the long-term safety of the vaccine for any age group is not known.

Supplement 1: Risk of Severe Systemic Reactions
from the Pfizer COVID-19 Vaccine
The Pfizer COVID-19 vaccine clinical trial found that the vaccine causes severe (grade 3) systemic reactions, which include fever greater than 102.1° F; vomiting that requires IV hydration; diarrhea of six or more loose stools in 24 hours; and severe fatigue, severe headache, severe muscle pain, or severe joint pain that prevents daily activity. Trial data21 demonstrate the following:
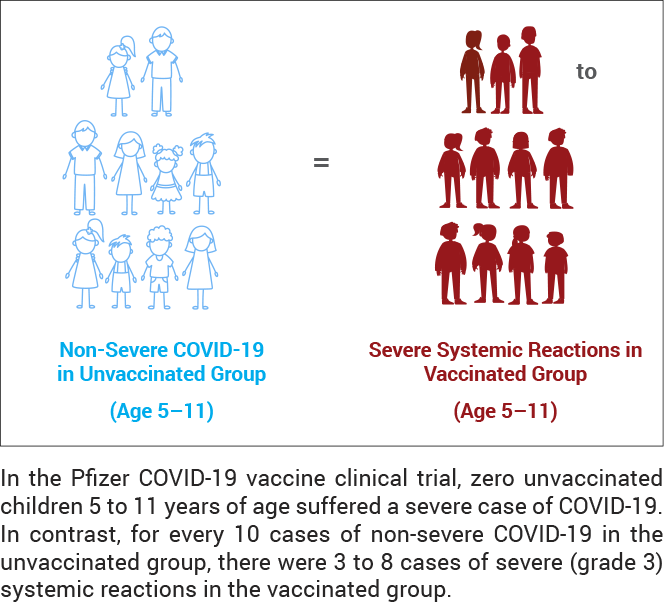
Children 5 to 11 years of age
- A range of 1 in 59 to 1 in 143 vaccinated children suffered severe systemic reactions within seven days of the second dose.
- There was a 6 to 7 times greater risk of a severe systemic reaction from the vaccine.
- There were 3 to 8 cases of severe systemic reactions observed in the vaccinated group for every 10 cases of non-severe COVID-19 in the unvaccinated group.
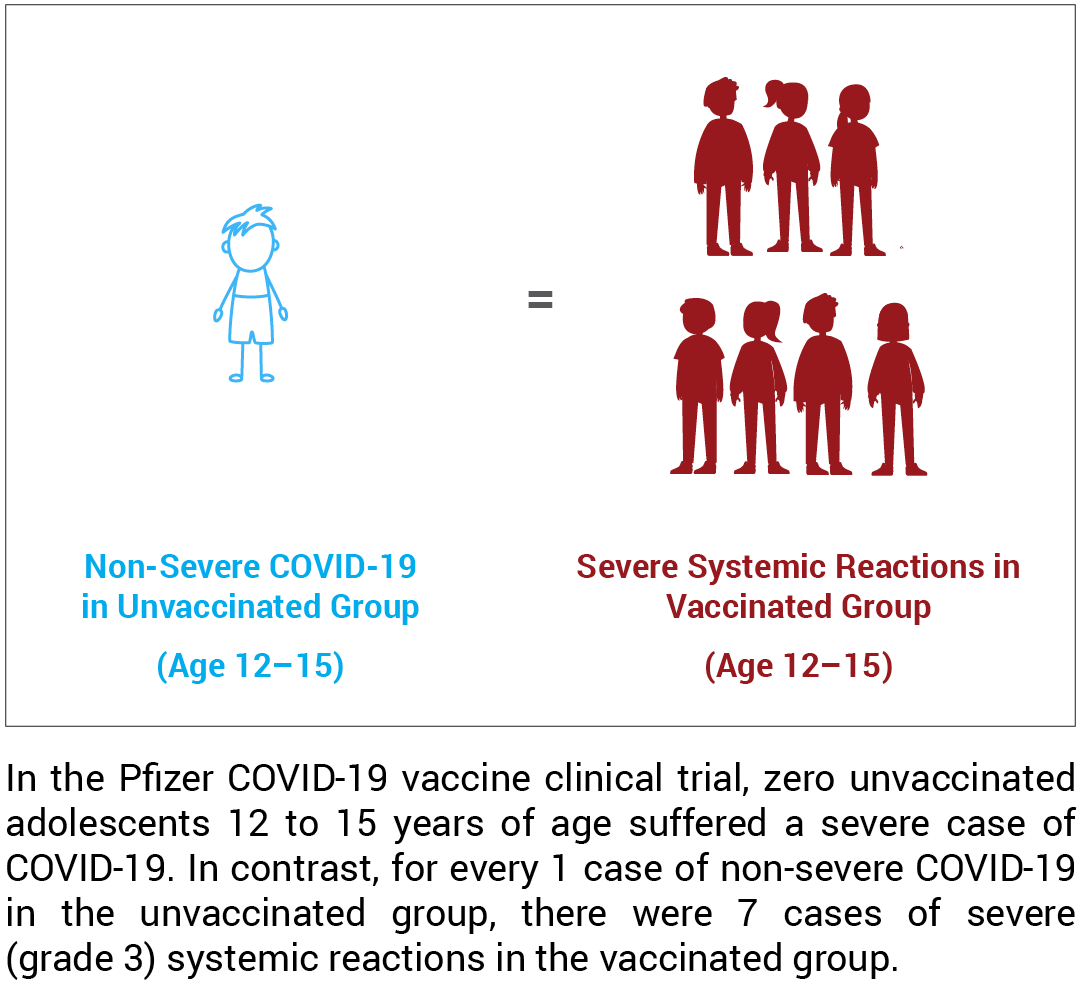
Adolescents 12 to 15 years of age
- 1 in 9 vaccinated adolescents suffered severe systemic reactions within seven days of the second dose.
- There was a 6 times greater risk of a severe systemic reaction from the vaccine.
- There were 7 times more severe systemic reactions observed in the vaccinated group than non-severe COVID-19 cases in the unvaccinated group.
People 16 to 55 years of age
- A range of 1 in 6 to 1 in 19 vaccinated subjects suffered severe systemic reactions within seven days of the second dose.
- There was an 8 to 10 times greater risk of a severe systemic reaction from the vaccine.
- There were 1 to 3 times more severe systemic reactions observed in the vaccinated group than COVID-19 cases of any severity in the unvaccinated group.
People 56 years or olders
- A range of 1 in 14 to 1 in 30 vaccinated subjects suffered severe systemic reactions within seven days of the second dose.
- There was a 9 to 11 times greater risk of a severe systemic reaction from the vaccine.
- There were 1 to 2 times more severe systemic reactions observed in the vaccinated group than COVID-19 cases of any severity in the unvaccinated group.
REFERENCES
-
Pfizer. New York (NY): Pfizer Inc. Vaccine information fact sheet for recipients and caregivers about Comirnaty (COVID-19 vaccine, mRNA) and Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) for use in individuals 12 years of age and older; revised 2021 Nov 19. https://www.fda.gov/media/153716/download.
-
Hinton, Denise M. (U.S. Food and Drug Administration). Letter to: Elisa Harkins (Pfizer Inc.). 2021 May 10. https://www.fda.gov/media/144412/download.
-
U.S. Food and Drug Administration, Division of Vaccines and Related Product Applications (DVRPA) Office of Vaccines Research and Review (OVRR). Washington, D.C.: U.S. Department of Health and Human Services. Summary basis for regulatory action; 2021 Nov 8: 7, 8. https://www.fda.gov/media/151733/download.
-
U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: EUA amendment request for Pfizer-BioNTech COVID-19 vaccine for use in children 5 through 11 years of age. Vaccines and Related Biological Products Advisory Committee Meeting: October 26, 2021: 20, 24, 26-29. https://www.fda.gov/media/153447/download.
-
Pfizer. Pfizer-BioNTech COVID-19 vaccine (BNT162, PF-07302048): Vaccines and Related Biological Products Advisory Committee briefing document. Meeting date: 10 December 2020. 2020 Nov 30: 38,46. https://www.fda.gov/media/144246/download.
-
Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU, C4591001 Clinical Trial Group. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv 2021 Jul 28:21261159. https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1.
-
U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Pfizer-BioNTech COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 10, 2020. Table 2: efficacy populations, treatment groups as randomized; 18. https://www.fda.gov/media/144245/download.
-
U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Pfizer-BioNTech COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 10, 2020:14,16,17,20,24,30,31,40,46,48. https://www.fda.gov/media/144245/download.
-
Shitrit P, Zuckerman NS, Mor O, Gottesman BS, Chowers M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill. 2021 Sep;26(39). https://pubmed.ncbi.nlm.nih.gov/34596015/.
-
Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, Gallagher GR, Fink T, Madoff LC, Gabriel SB, MacInnis B, Park DJ, Siddle KJ, Harik V, Arvidson D, Brock-Fisher T, Dunn M, Kearns A, Laney AS. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 6;70(31):1059-62. https://www.cdc.gov/mmwr/volumes/70/wr/mm7031e2.htm?s_cid=mm7031e2_w.
-
Pfizer. New York (NY): Pfizer Inc. Comirnaty (COVID-19 vaccine, mRNA) suspension for injection, for intramuscular use; revised 2021 Aug. https://www.fda.gov/media/151707/download.
-
Pfizer. New York (NY): Pfizer Inc. Fact sheet for healthcare providers administering vaccine (vaccination providers); revised 2021 Nov 19: 17, 48. https://www.fda.gov/media/153713/download.
-
U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) Office of Vaccines Research and Review (OVRR). Washington, D.C.: U.S. Department of Health and Human Services. Emergency use authorization (EUA) amendment for an unapproved product: review memorandum; 2021 Apr 9: 23, 39. https://www.fda.gov/media/148542/download.
-
U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) Office of Vaccines Research and Review (OVRR). Washington, D.C.: U.S. Department of Health and Human Services. Emergency use authorization (EUA) amendment for an unapproved product: review memorandum; 2021 Sep 21: 4, 29, 32. https://www.fda.gov/media/152432/download.
-
U.S. Food and Drug Administration. Washington, D.C.: U.S. Department of Health and Human Services. Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters. 2021 Nov 19 [cited 2021 Nov 25]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters.
-
U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Application for licensure of a booster dose for Comirnaty (COVID-19 Vaccine, mRNA). Vaccines and Related Biological Products Advisory Committee Meeting: September 17, 2021. 4, 7. https://www.fda.gov/media/152176/download.
-
Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021 May;174(5):655-62. https://doi.org/10.7326/M20-6976.
-
Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. COVID-19 — Disease Information Statement (DIS). Aug 2021. https://physiciansforinformedconsent.org/covid-19/.
-
Subramanian SV, Kumar A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol. 2021 Sep 30:1-4. https://pubmed.ncbi.nlm.nih.gov/34591202/.
-
Pfizer. A phase 1/2/3 study to evaluate the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals: 61, 62. https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf.
For children 5 to 11 years of age, the overall incidence of severe systemic reactions within seven days of the second dose was 0.7% to 1.7%, or 1 in 143 to 1 in 59, respectively, in the vaccinated group compared to 0.1% to 0.3% in the unvaccinated group, resulting in a 6 to 7 times greater risk of a severe systemic reaction from the vaccine.4 In addition, the incidence of COVID-19 in the unvaccinated group was 2.2%;4 therefore, there were 0.3 to 0.8 times as many severe systemic reactions observed in the vaccinated group than there were non-severe cases of COVID-19 in the unvaccinated group.
For adolescents 12 to 15 years of age, the overall incidence of severe systemic reactions within seven days of the second dose was 10.7%, or 1 in 9, in the vaccinated group compared to 1.9% in the unvaccinated group, resulting in a nearly 6 times greater risk of a severe systemic reaction from the vaccine.22,23 In addition, the incidence of COVID-19 in the unvaccinated group was 1.6%;24 therefore, there were almost 7 times more severe systemic reactions observed in the vaccinated group than there were non-severe cases of COVID-19 cases in the unvaccinated group.
For subjects 16 to 55 years of age, the overall incidence of severe systemic reactions within seven days of the second dose was 5.3% to 16.4%, or 1 in 19 to 1 in 6, respectively, in the vaccinated group compared to 0.7% to 1.6% in the unvaccinated group, resulting in an 8 to 10 times greater risk of a severe systemic reaction from the vaccine.11 In addition, the incidence of COVID-19 in the unvaccinated group was 4.9%;6 therefore, there were 1 to 3 times more severe systemic reactions observed in the vaccinated group than there were COVID-19 cases of any severity in the unvaccinated group.
For subjects 56 years or older, the overall incidence of severe systemic reactions within seven days of the second dose was 3.3% to 7.3%, or 1 in 30 to 1 in 14, respectively, in the vaccinated group compared to 0.3% to 0.8% in the unvaccinated group, resulting in a 9 to 11 times greater risk of a severe systemic reaction from the vaccine.11 In addition, the incidence of COVID-19 in the unvaccinated group was 3.2%;6 therefore, there were 1 to 2 times more severe systemic reactions observed in the vaccinated group than there were COVID-19 cases of any severity in the unvaccinated group.
-
Wallace M. Grading of recommendations, assessment, development, and evaluation (GRADE): Pfizer-BioNTech COVID-19 Vaccine. COVID-19 Vaccines Work Group of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. 2021 May 12: 24, 25. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/03-COVID-Wallace-508.pdf.
-
Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Grading of recommendations, assessment, development, and evaluation (GRADE): Pfizer-BioNTech COVID-19 vaccine for persons aged 12-15 years; [cited 2021 May 22]. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-pfizer-biontech-vaccine-12-15-years.html#table03d.
-
Pfizer. New York (NY): Pfizer Inc. Fact sheet for healthcare providers administering vaccine (vaccination providers); revised 2021 Nov 19. Table 11: vaccine efficacy – first COVID-19 occurrence from 7 days after dose 2: without evidence of infection and with or without evidence of infection prior to 7 days after dose 2 – blinded placebo-controlled follow-up period, adolescents 12 through 15 years of age evaluable efficacy (7 days) population; 48. https://www.fda.gov/media/153713/download.
-
Clark T. Anaphylaxis following mRNA COVID-19 vaccine receipt. COVID-19 Vaccines Work Group of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. 2020 Dec 19; [cited 2021 Mar 16]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-Clark-508.pdf.
-
Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Weekly updates by select demographic and geographic characteristics: provisional death counts for coronavirus disease (COVID-19); [cited 2021 Nov 3]. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#AgeAndSex.
These statements are intended for informational purposes only and should not be construed as personal medical advice.
© 2022 Physicians for Informed Consent, an independent 501(c)(3) nonprofit educational organization. All rights reserved. Updated Jan 2022.
