IPV Vaccine (Polio): Is It Safer Than Polio?
1. WHAT IS THE IPV VACCINE?
IPV (inactivated poliovirus) vaccine is a polio vaccine that was first introduced in the U.S. in 1955. IPV vaccine has significantly reduced the incidence of reported (i.e., noticeable) cases of polio infections; however, the vaccine does not prevent asymptomatic infection or the spread of polio.1,2 OPV (oral poliovirus) vaccine is an attenuated live-virus vaccine that is no longer used in the U.S. due to the risk of vaccine-associated paralytic polio.3
 2. WHAT ARE SIDE EFFECTS OF THE IPV VACCINE?
2. WHAT ARE SIDE EFFECTS OF THE IPV VACCINE?
Common side effects of the IPV vaccine include fever, loss of appetite, vomiting, and fatigue.4 A more serious potential side effect is seizure, which may occur in about 1 in 829 children vaccinated with IPV.5-8 Although severe adverse events, such as Guillain-Barré syndrome and sudden infant death syndrome, have been observed following IPV vaccination, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relation between IPV” and those conditions.9 Additionally, the manufacturer’s package insert states, “Long-term studies in animals to evaluate carcinogenic potential or impairment of fertility have not been conducted” for the IPV vaccine.4
![]()
3. HOW ARE RISKS OF VACCINE SIDE EFFECTS MEASURED?
Methods to measure vaccine risks include surveillance systems, clinical trials, and epidemiological studies.
![]()
4. HOW ACCURATE IS SURVEILLANCE OF ADVERSE EVENTS FROM THE IPV VACCINE?
The government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 99 cases of permanent injury and death from the IPV vaccine are reported to VAERS annually.10 However, VAERS is a passive reporting system — authorities do not actively search for cases and do not actively remind doctors and the public to report cases. These limitations can lead to significant underreporting.11 The Centers for Disease Control and Prevention (CDC) states, “VAERS receives reports for only a small fraction of actual adverse events.”12 Indeed, as few as 1% of serious side effects from medical products are reported to passive surveillance systems.13 In addition, VAERS reports are not proof that a side effect occurred, as the system is not designed to thoroughly investigate all cases.14 As a result, VAERS does not provide an accurate count of IPV vaccine side effects.
![]()
5. HOW ACCURATE ARE CLINICAL TRIALS OF THE IPV VACCINE?
The CDC states, “Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.”11 In the pre-vaccine era, annually permanent injury from polio occurred in about 1 in 190,000 children under age 10 at normal risk (i.e., had tonsils and rested after feeling sick).15 Therefore, about 1 in 19,000 such children suffered permanent injury from polio in a 10-year span. A few thousand subjects in clinical trials are not enough to prove that the IPV vaccine causes less permanent injury than polio in children at normal risk (Fig. 1).
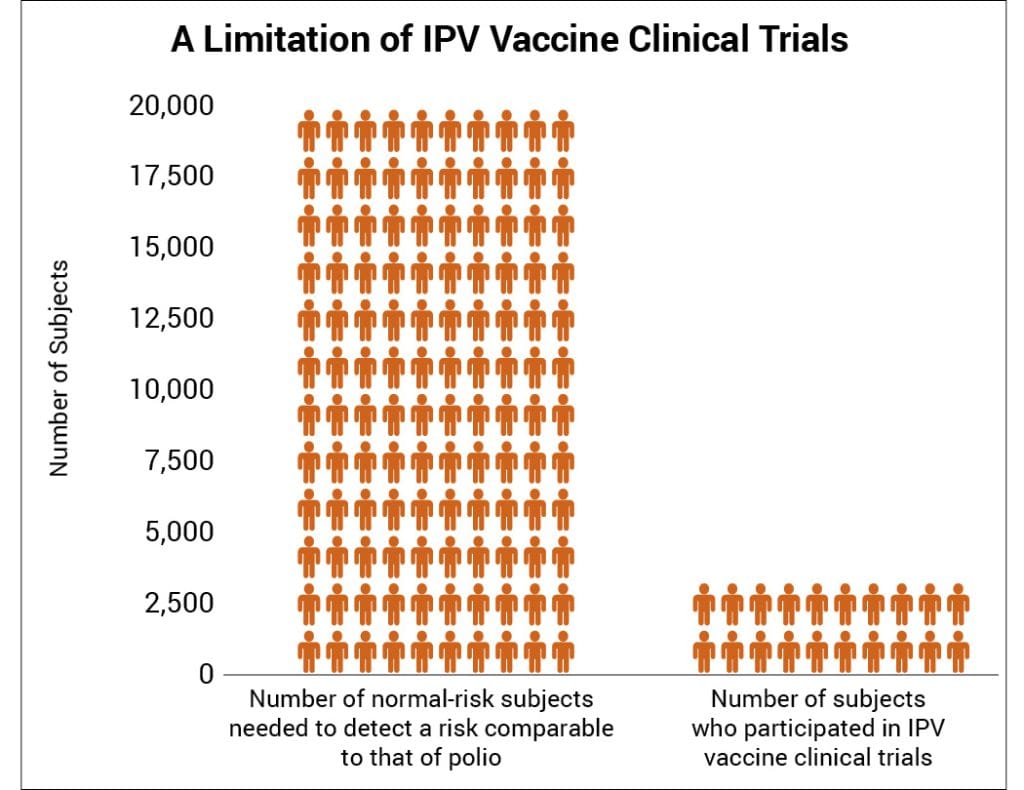
Figure 1: There are not enough subjects in clinical trials to prove that the IPV vaccine poses less risk than polio for children with tonsils who rest after feeling sick.
6. HOW ACCURATE ARE EPIDEMIOLOGICAL STUDIES OF THE IPV VACCINE?
Epidemiological studies are hindered by the effects of chance. For example, a study published in the Journal of the American Medical Association (JAMA) involving about 379,000 children looked for an association between an IPV-containing vaccine and certain adverse events.16 Although the study found no association between the IPV-containing vaccine and the adverse events, it did not rule out the possibility that the vaccine increases the risk of an adverse event that leads to permanent injury by up to 56%. Consequently, the study did not rule out the possibility that such adverse events might occur up to 38 times more often than permanent injury from polio in children at normal risk: 1 in 500 compared to 1 in 19,000 (Fig. 2 and Table 1). The range of possibilities found in the study makes the result inconclusive; even large epidemiological studies are not accurate enough to prove that the IPV vaccine causes less permanent injury or death than polio in children at normal risk.
![]()
7. IS THE IPV VACCINE SAFER THAN POLIO?
It has not been proven that the IPV vaccine is safer than polio. The vaccine package insert raises questions about safety testing for cancer and impaired fertility. Although VAERS tracks some adverse events, it is too inaccurate to measure against the risk of polio. Clinical trials do not have the ability to detect less common adverse reactions, and epidemiological studies are limited by the effects of chance. Safety studies of the IPV vaccine are lacking in statistical power. A review of IPV vaccine safety studies conducted by the IOM found that the evidence was inadequate to rule out the possibility that IPV vaccination leads to Guillain-Barré syndrome and sudden infant death syndrome.9 Because permanent sequelae (aftereffects) from polio are so rare in children at normal risk, the level of accuracy of the research studies available is insufficient to prove that the IPV vaccine causes less permanent injury or death than polio in normal-risk children.
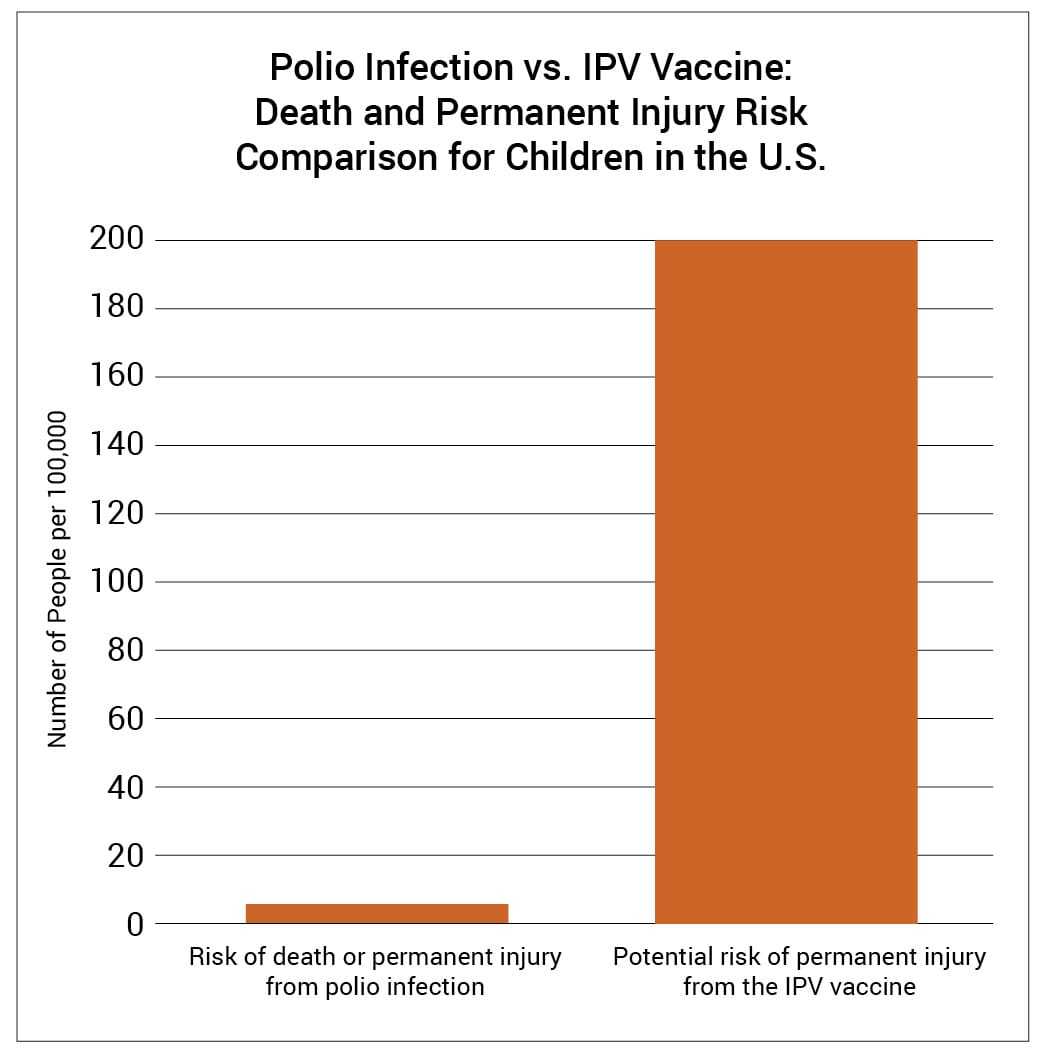
Figure 2: A study published in JAMA did not rule out the possibility that an IPV-containing vaccine can cause an adverse event leading to permanent injury 38 times more often than polio can cause permanent injury in U.S. children at normal risk.

REFERENCES
- Cuba IPV Study Collaborative Group. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N Engl J Med. 2007 Apr 12;356(15):1536-44. http://www.nejm.org/doi/full/10.1056/NEJMoa054960?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub=pubmed.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. U.S. National Authority for Containment of Poliovirus: interim guidance for potentially infectious materials; [cited 2024 Nov 2]. https://stacks.cdc.gov/view/cdc/138966.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, editors. Washington, D.C.: Public Health Foundation; 2021. 279-81. https://physiciansforinformedconsent.org/cdc-pink-book-14th-edition-2021/.
- Sanofi Pasteur SA. Marcy L’Etoile, France: Sanofi Pasteur SA. IPOL (Poliovirus Vaccine Inactivated); 2022 May [cited 2023 Mar 16]. https://www.fda.gov/media/75695/download.
- Between 2000 and 2004, the Vaccine Adverse Event Reporting System (VAERS) received 477 reports of seizure-related adverse events involving IPV in children <1 year of age.6 In the same time period, VAERS received 618 reports of seizure-related adverse events involving measles vaccine in children 1-2 years of age.7 This suggests that seizures from IPV vaccine may occur 77.2% as often as seizures from MMR vaccine, 77.2% of 1 in 6408 or about 1 in 829.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2023 March 20]. https://wonder.cdc.gov/vaers.html. Query for seizure-related events involving IPV vaccine, 2000-2004.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2022 October 20]. https://wonder.cdc.gov/vaers.html. Query for seizure-related events involving all measles-containing vaccines, 2000-2004.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004 Jul 21;292(3):356. https://jamanetwork.com/journals/jama/fullarticle/199117.
- Institute of Medicine (IOM). Adverse events associated with childhood vaccines: evidence bearing on causality. Washington, D.C.: National Academy Press; 1994. 204,206. https://pubmed.ncbi.nlm.nih.gov/25144097/.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2023 March 27]. https://wonder.cdc.gov/vaers.html. Query for death and permanent disability involving IPV vaccine, 2000-2004.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Miller ER, Haber P, Hibbs B, Broder K. Chapter 21: surveillance for adverse events following immunization using the Vaccine Adverse Event Reporting System (VAERS). Atlanta: Centers for Disease Control and Prevention; 2011. 1,2,8. https://physiciansforinformedconsent.org/cdc-manual-for-the-surveillance-of-vaccine-preventable-diseases-5th-ed-chpt21-surv-adverse-events-2011.
- Centers for Disease Control and Prevention, Food and Drug Administration. Washington D.C.: U.S. Department of Health and Human Services. Guide to interpreting VAERS data; [cited 2022 May 28]. https://vaers.hhs.gov/data/dataguide.html.
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993 Jun 2;269(21):2765-8. https://www.sciencedirect.com/science/article/abs/pii/0163834394900515?via%3Dihub.
- Centers for Disease Control and Prevention. Washington D.C.: U.S. Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2022 May 28]. https://wonder.cdc.gov/vaers.html.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4):561. https://pubmed.ncbi.nlm.nih.gov/33003480/.
- Sun Y, Christensen J, Hviid A, Li J, Vedsted P, Olsen J, Vestergaard M. Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type b. JAMA. 2012 Feb 22;307(8):823-31. https://jamanetwork.com/journals/jama/fullarticle/1355993.
Published 2023 Aug; updated 2024 Dec