Haemophilus Influenzae Type B (Hib) – Disease Information Statement (DIS)
Haemophilus Influenzae Type B (Hib)
What You Need to Know
What Is Hib?
Haemophilus influenzae type b (Hib) is a bacterial infection.
- Most Hib infections are asymptomatic (have no symptoms).1
- Before the Hib mass vaccination program was introduced, most children contracted Hib and obtained immunity by age 5-6.2
- A small proportion of Hib infections lead to invasive disease. Invasive Hib disease symptoms include fever, decreased mental status, stiff neck, swelling of the epiglottis (a tissue in the throat), or joint, ear, or skin pain.2
- Invasive Hib disease can result in deafness, intellectual impairment, or death; however, more than 90% of cases recover without disability.3


What Are the Risks?
Prior to 1985, before the introduction of the Hib vaccine, invasive Hib was a disease of low incidence. Cases of invasive Hib occurred in about 1 in 68,000 (0.001%) in the U.S. population.4
Breastfeeding exclusively (i.e., breastfeeding alone without formula) can prevent invasive Hib infections. The majority of invasive Hib infections occur in children who are not breastfed exclusively for 13 weeks or more.5


Before the introduction of the Hib vaccine, annually about 1 in 143,000 or 0.0007% of children under age 5 who were breastfed exclusively for 13 weeks or more contracted invasive Hib that was fatal or led to permanent disability (Fig. 1).3
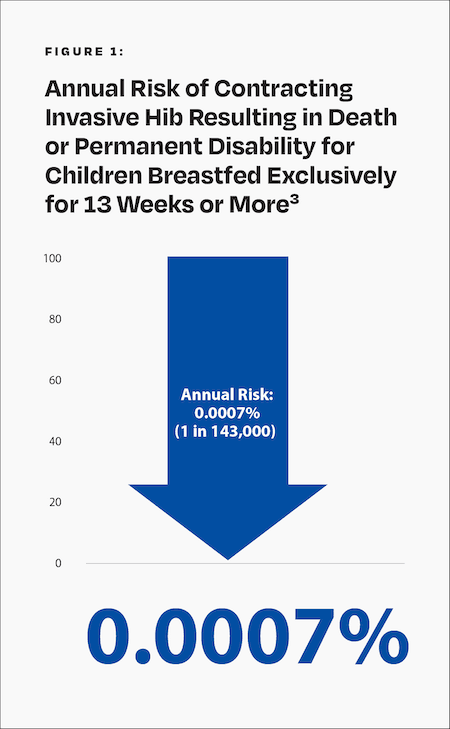
What Treatments Are Available?
Invasive Hib can be treated with antibiotics such as cephalosporin (cefotaxime or ceftriaxone), chloramphenicol, and ampicillin.2

What About the Hib Vaccine?
The Hib vaccine was introduced in the U.S. in 1985. It has significantly reduced the incidence of reported (i.e., noticeable) cases of Hib infections in children; however, studies have observed that mass vaccination may lead to an increase in the prevalence of non-type b Haemophilus influenzae strains.6,7
The manufacturer’s package insert contains information about vaccine ingredients, adverse reactions, and vaccine evaluations. For example, “PedvaxHIB has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility.”8 Furthermore, the risk of permanent disability or death from the Hib vaccine has not been proven to be less than that of Hib (Fig. 2).9
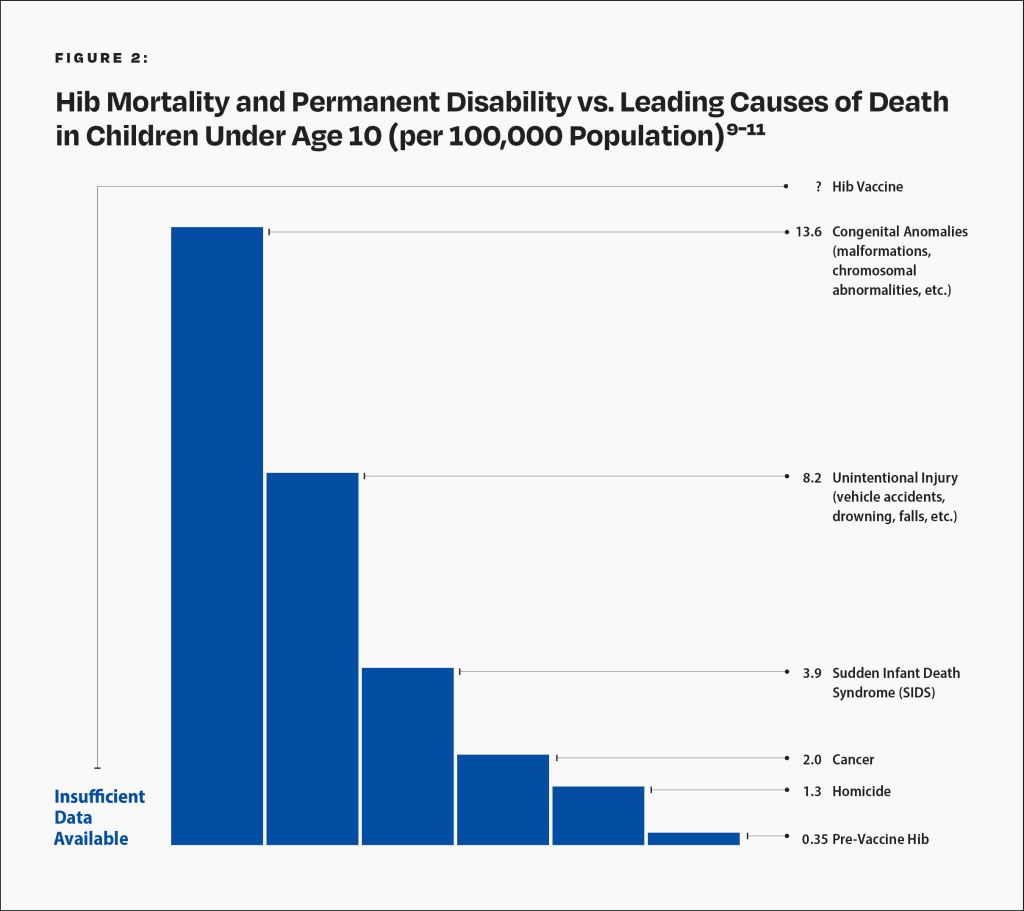
This graph shows the Hib death and permanent disability rate of children under age 10 who were breastfed exclusively for 13 weeks or more before the vaccine was introduced and compares it to the leading causes of death in children under age 10 today. Hence, in the pre-vaccine era, the Hib death and permanent disability rate per 100,000 was 0.35 for children under age 10 who were breastfed exclusively for 13 weeks or more. In 2015, the death rate per 100,000 for homicide was 1.3, followed by cancer (2.0), SIDS (3.9), unintentional injury (8.2), and congenital anomalies (13.6). The rate of death or permanent injury from the Hib vaccine is unknown because the research studies available are not able to measure it with sufficient accuracy.
What Treatments Are Available?
Invasive Hib can be treated with antibiotics such as cephalosporin (cefotaxime or ceftriaxone), chloramphenicol, and ampicillin.2

What About the HIB Vaccine?
The Hib vaccine was introduced in the U.S. in 1985. It has significantly reduced the incidence of reported (i.e., noticeable) cases of Hib infections in children; however, studies have observed that mass vaccination may lead to an increase in the prevalence of non-type B Haemophilus influenzae strains.6,7
The manufacturer’s package insert contains information about vaccine ingredients, adverse reactions, and vaccine evaluations. For example, “PedvaxHIB has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility.”8 Furthermore, the risk of permanent disability or death from the Hib vaccine has not been proven to be less than that of Hib (Fig. 2).9
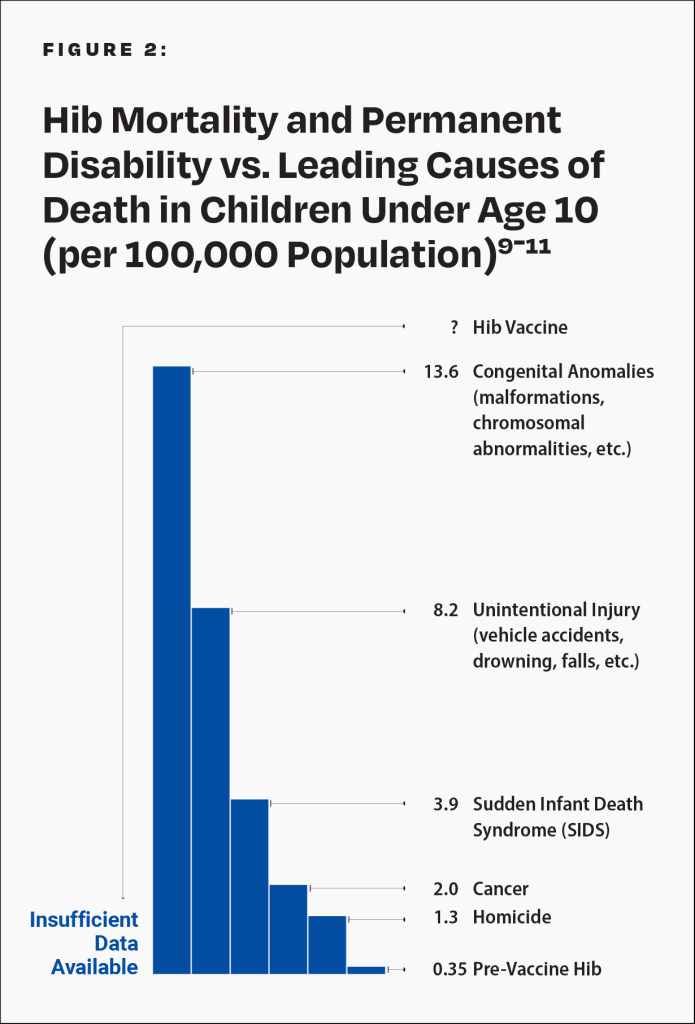
This graph shows the Hib death and permanent disability rate of children under age 10 who were breastfed exclusively for 13 weeks or more before the vaccine was introduced and compares it to the leading causes of death in children under age 10 today. Hence, in the pre-vaccine era, the Hib death and permanent disability rate per 100,000 was 0.35 for children under age 10 who were breastfed exclusively for 13 weeks or more. In 2015, the death rate per 100,000 for homicide was 1.3, followed by cancer (2.0), SIDS (3.9), unintentional injury (8.2), and congenital anomalies (13.6). The rate of death or permanent injury from the Hib vaccine is unknown because the research studies available are not able to measure it with sufficient accuracy.
References
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, editors. Washington, D.C.: Public Health Foundation; 2021. 112. https://physiciansforinformedconsent.org/cdc-pink-book-14th-edition-2021/.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Hamborsky J, Kroger A, Wolfe S, editors. Washington, D.C.: Public Health Foundation; 2015. 120-122. https://physiciansforinformedconsent.org/cdc-pink-book-13th-edition-2015/.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4):7, Suppl 14. https://pubmed.ncbi.nlm.nih.gov/33003480/.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4):7. https://pubmed.ncbi.nlm.nih.gov/33003480/; during 1980–1984, there were 3,400 annual cases of invasive Hib out of a population of 230 million (1 in 68,000).
- Silfverdal SA, Bodin L, Hugosson S, Garpenholt O, Werner B, Esbjorner E, Lindquist B, Olce P. Protective effect of breastfeeding on invasive Haemophilus influenzae infection: a case-control study in Swedish preschool children. Int J Epidemiol. 1997 Apr;26(2):446. https://pubmed.ncbi.nlm.nih.gov/9169183/.
- Perdue DG, Bulkow LR, Gellin BG, Davidson M, Petersen KM, Singleton RJ, Parkinson AJ. Invasive Haemophilus influenzae disease in Alaskan residents aged 10 years and older before and after infant vaccination programs. JAMA. 2000 Jun 21;283(23):3089-94. https://pubmed.ncbi.nlm.nih.gov/10865303/.
- Langereis JD, de Jonge MI. Invasive disease caused by nontypeable Haemophilus influenzae. Emerg Infect Dis. 2015 Oct;21(10):1711-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4593434/.
- Merck. Rahway (NJ): Merck and Co., Inc. Liquid PedvaxHIB; revised 2023 Apr [cited 2024 May 4]. 6. https://www.merck.com/product/usa/pi_circulars/p/pedvax_hib/pedvax_pi.pdf.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Haemophilus influenzae type b (Hib) — vaccine risk statement (VRS). Hib vaccine: is it safer than Hib? 2024 Aug. https://physiciansforinformedconsent.org/hib-vrs.
- Magno H, Golomb B. Measuring the benefits of mass vaccination programs in the United States. Vaccines. 2020 Sep 29;8(4)Suppl:14. https://pubmed.ncbi.nlm.nih.gov/33003480/; the study observed that annually about 1 in 143,000 of children under age 5 who were breastfed exclusively for 13 weeks contracted a case of Hib resulting in death or permanent disability. Therefore, because there are twice as many children under age 10 as there are children under age 5, the rate relative to children under age 10 is 1 in 286,000.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. 10 leading causes of death by age group, United States—2015. https://physiciansforinformedconsent.org/cdc-leading-causes-of-death-age-group-2015/.
Published 2024 Aug
