Vaccine safety studies are intended to prove that the risk of a vaccine is less than the risk of the disease. However, the vaccine safety studies for the diphtheria, tetanus, and pertussis (DTaP); polio (IPV); Haemophilus influenzae type b (Hib); varicella (chicken pox); hepatitis B; and measles, mumps, and rubella (MMR) vaccines do not have the statistical power to detect risk levels as low as the 10 diseases they target.
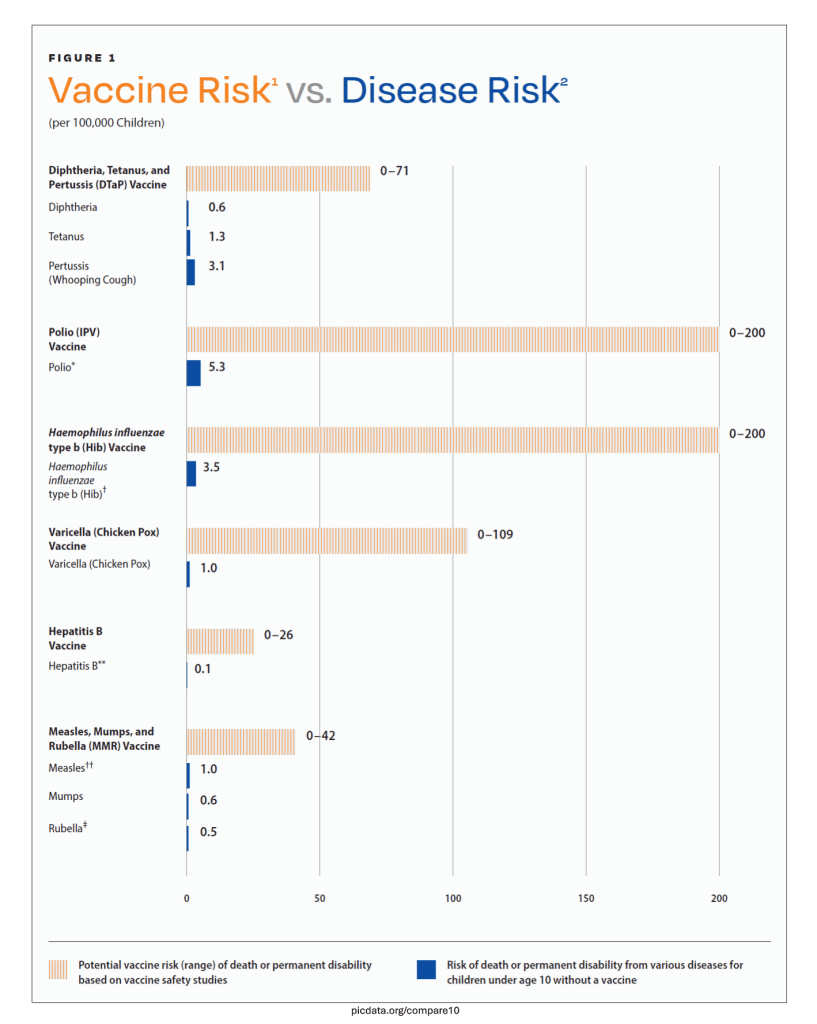
The blue bars on the graph show the risk of death or permanent disability from diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b (Hib), hepatitis B, measles, mumps, rubella, and varicella (chicken pox), in children under age 10, without a vaccine. The orange bars show the range of the potential vaccine risk of death or permanent disability that has not been ruled out by vaccine safety studies.
Compare, for example, the risk of diphtheria, tetanus, and pertussis (DTaP) to the risk of the DTaP vaccine:
• Disease Risk — Risk from diphtheria, tetanus, and pertussis is 0.6 in 100,000, 1.3 in 100,000, and 3.1 in 100,000, respectively.
• Vaccine Risk — Because the smallest risk that can be detected by DTaP vaccine safety studies is 71 in 100,000, the actual risk from the DTaP vaccine ranges from 0 to 71 in 100,000. Therefore, it is not known whether the actual vaccine risk is less than or greater than the disease risk (see note).
The range of possibilities presented in vaccine safety studies makes the results inconclusive when compared to the known risks of diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b (Hib), hepatitis B, measles, mumps, rubella, and varicella (chicken pox). Consequently, vaccine safety studies do not rule out the possibility that the vaccines cause greater death or permanent disability than the diseases they target.
Conclusion: Since the vaccines for the 10 diseases above may cause more death or permanent disability than the diseases they target, they have not been proven safer than the diseases they target.
Note: Statistical power is defined by the minimum risk that can be detected by a safety study based on the width of the confidence interval. The width of the confidence interval is a function of the sample size and the prevalence of the condition being studied.
¹Vaccine Risk Statements (VRS) for DTaP, IPV, Hib, varicella, hepatitis B, and MMR.
²Disease Information Statements (DIS) for diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b (Hib), hepatitis B, measles, mumps, rubella, and varicella (chicken pox). The DISs present annual risks, while the disease risks in the figure above use those annual risks to derive the risk for the entire life of a 10-year-old child, as it is done in the VRSs.
*This rate only considers the child population with tonsils that typically rests after feeling sick, about 84% of the child population.
†This rate only considers the child population that was breastfed alone without formula for at least 13 weeks, about 47% of the child population.
**This rate only considers the child population that is not born to an infected mother and does not live with an infected individual or in a community with a large number of infected individuals, about 95% of the child population.
††This rate only considers the child population with normal levels of vitamin A, about 95% of the child population.
‡This rate only considers the child population that contracts rubella after birth; about 89% of rubella cases are not at risk of infecting a fetus through pregnancy.
